JOURNAL 1853
Organic Communications
VOLUME & ISSUE
Year: 2020 Issue: 4 October-December
Year: 2020 Issue: 4 October-December
PAGES
p.175 - 183
p.175 - 183
STATISTICS
Viewed 3088 times.
Viewed 3088 times.
GRAPHICAL ABSTRACT
ABSTRACT
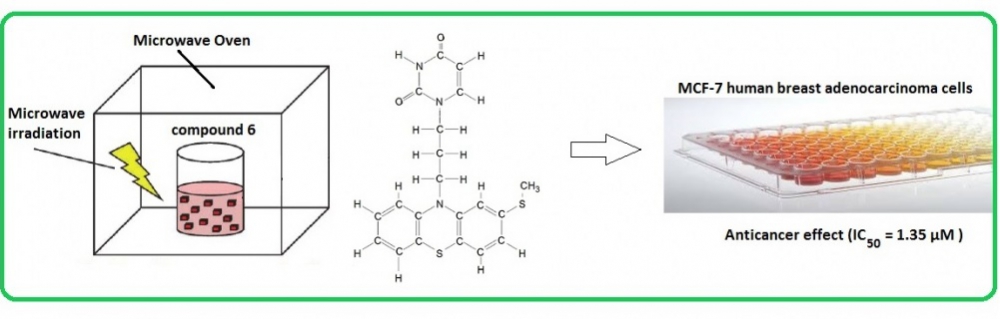
Herein, a facile procedure for microwave synthesis and NMR characterization of phenothiazine derivatives 10-(3-hydroxypropyl)-2-(methylsulphanyl)-10H-phenothiazine (3) (25% yield) and 1-[3-(2-methylsulfanyl-10H-phenothiazine-10-yl)-propyl]pyrimidine-2,4-(1H,3H)-dione (6) (67% yield) is described. After successful microwave synthesis steps, MTT cytotoxicity experiments gave rise to greater anticancer effect of compound 6 in MCF-7 human breast adenocarcinoma cell line (IC50= 1.35 µM) as compared to literature values for tamoxifen (IC50= 8.3 µM) and doxorubicin (IC50= 27 µM).
KEYWORDS- Microwave synthesis
- phenothiazine derivatives
- 1-(3-(2-methylsulfanyl-10H-phenothiazine-10-yl)-prop-1-yl)pyrimidine-2,4(1H,3H)-dione
- NMR
- IC50
- MCF-7