JOURNAL 1959
Organic Communications
Year: 2021 Issue: 2 April-June
p.121 - 132
Viewed 3261 times.
-
Priyadarsini Pullagura

-
Madhava Rao Vallabhaneni

-
Hanumatha Rao Addanki

-
Ch. Subramanyam

-
Ranganayakulu Yenisetty

GRAPHICAL ABSTRACT
ABSTRACT
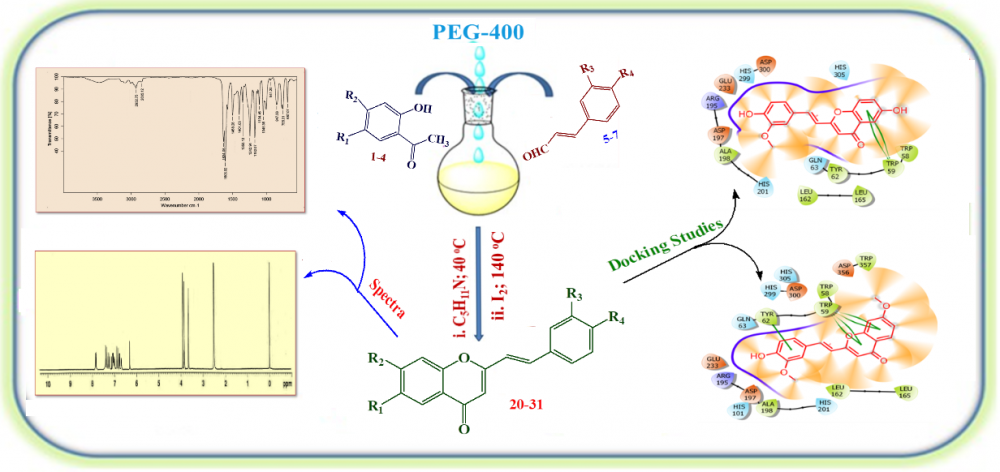
2-Styrylchromones have been synthesized successfully by using eco-friendly, recoverable and reusable PEG-400 as reaction medium in the presence of the catalytic amount of piperidine under warm conditions through 1,5-diphenylpenta-2,4-dien-1-ones as intermediates followed by oxidative cyclization with iodine in the same reaction vessel starting from 2-hydroxyacetophenones and cinnamaldehydes. The synthesized compounds were characterized by some physical methods and spectral data like IR, NMR, and LCMS etc. In silico molecular docking study was performed for all the synthesized compounds to know their ability in inhibiting pancreatic α-amylase enzyme. In this study, the compounds 24, 25, 26 & 28 with binding energies, -8.7, -8.8, -8.6 and -8.4 kcal/mol respectively were found to be more active amongst the synthesized when compared with standard drug, acarbose (-8.2 kcal/mol).
KEYWORDS- 2-Styrylchromones,
- eco-friendly
- PEG-400
- molecular docking
- acarbose
- α-amylase