JOURNAL 1998
Organic Communications
Year: 2021 Issue: 2 April-June
p.133 - 143
Viewed 2954 times.
GRAPHICAL ABSTRACT
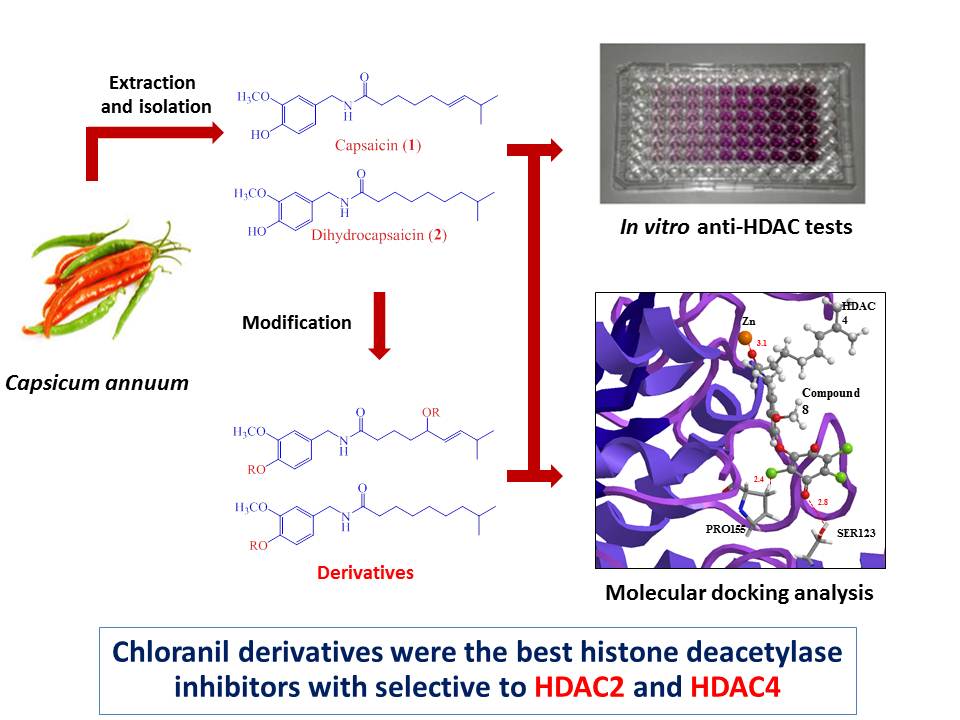
ABSTRACT
Capsaicin and dihydrocapsaicin were modified at phenolic moieties and allylic of double-bond to provide seven capsaicin and dihydrocapsaicin derivatives. Their structures were assigned by spectroscopic techniques (1D-NMR and MS). All compounds were evaluated as histone deacetylase inhibitors via in vitro fluorometric assay at 100 µM. The results revealed that the chloranil derivatives were found to be best histone deacetylase inhibitors among the tested compounds with 86% and 87% inhibitions. In addition, molecular docking experiments of the active compounds with representatives of class I (HDAC1, HDAC2, HDAC3 and HDAC8), class IIa (HDAC4 and HDAC7) and class IIb (HDAC6) HDAC isoforms displayed potential isoform-selective HDAC inhibitors. Those data show a new method for providing the isoform-selective histone deacetylase inhibitors from common natural products.
KEYWORDS- Red chili pepper
- Capsicum annuum
- histone deacetylase
- HeLa cell
- cytotoxiciy
- molecular docking