JOURNAL 2244
Organic Communications
Year: 2022 Issue: 1 January-March
p.1 - 31
Viewed 2931 times.
-
Darshini Patel

-
Umang Shah

-
Arya Patel

-
Sandip Patel

-
Mehul Patel

-
Ashish Patel

-
Swayamprakash Patel

-
Nilay Solanki

-
Nilesh Pandey

GRAPHICAL ABSTRACT
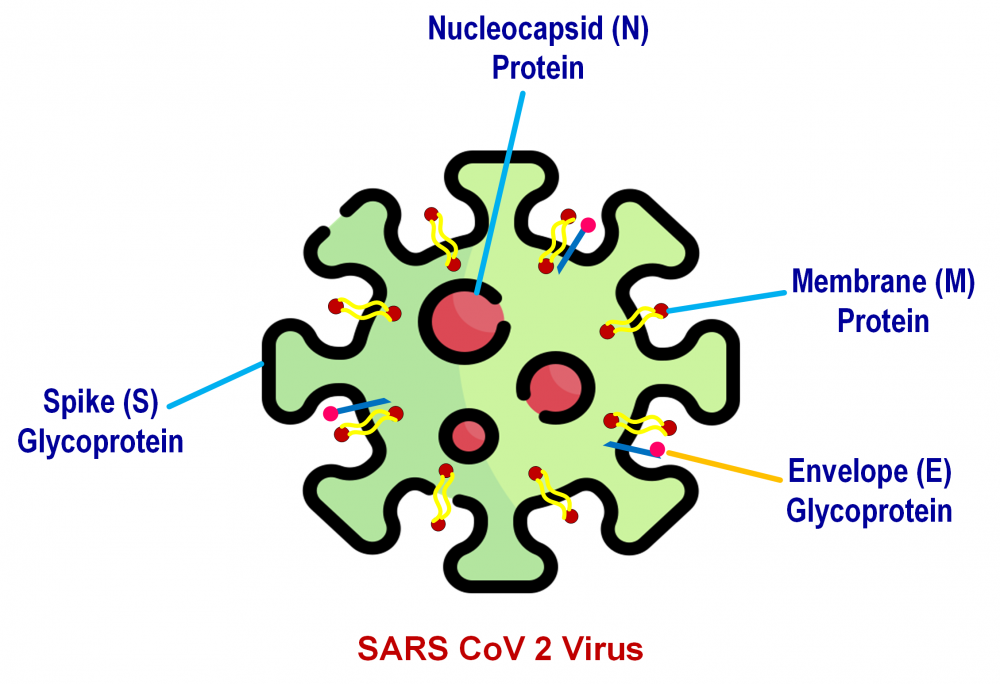
ABSTRACT
The SARS-CoV-2 virus, accountable for the COVID-19 pandemic, is now sweeping the globe. As a result, as this disease resists testing and adoption of new treatments, repositioning existing medications may provide a quick and appealing method with established safety, features, and dose used. They are not, however, specific or focused. However, numerous medications have been studied for their efficacy and safety in treatment of COVID-19, with the majority currently undergoing clinical trials. The goal is to rapidly expand novel preventative and therapeutic medications, as well as to apply preventive methods such as early patient identification, isolation, and treatment. Moreover, reducing transmission through physical contact is also important. In the fight against this dangerous disease, finding the proper treatment is crucial. This article summarizes several anti-malarial, anti-parasitic, monoclonal antibodies, immunosuppressant, and immunomodulating agents in clinical trials for COVID-19. The purpose of this article is to evaluate and explore the potential roles of several medications now utilized in COVID-19.
KEYWORDS- COVID-19
- SARS-CoV-2
- spike protein
- drug repurposing
- ACE
- coronavirus