JOURNAL 2307
Organic Communications
Year: 2022 Issue: 1 January-March
p.32 - 43
Viewed 2677 times.
GRAPHICAL ABSTRACT
ABSTRACT
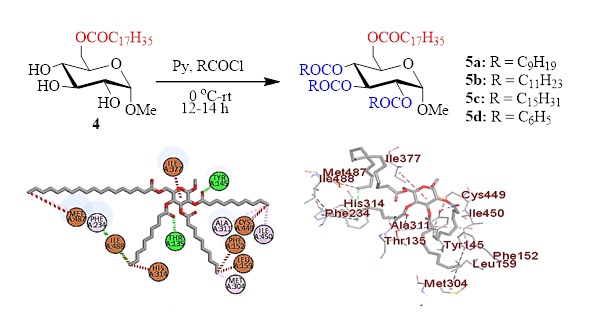
Sugar esters (SEs) with fatty acyl chains showed diverse applications including antimicrobial inhibition against multidrug-resistant (MDR) microorganisms. Thus, fatty acid esters, especially 6-O-stearoyl glucopyranoside ester was prepared by the treatment of glucopyranoside with unimolar stearoyl chloride at low temperature. The 6-O-stearoyl ester thus obtained was further modified to four newer 2,3,4-O-acyl esters to incorporate decanoyl, lauroyl, palmitoyl, and benzoyl chains in the glucopyranoside skeleton. Prediction of activity spectrum for substances (PASS) analyses suggested that these fatty acid esters are more prone to fungal pathogens compared to bacterial pathogens. Guided by PASS analyses in vitro antifungal activities were screened against four fungal pathogens, which supported the PASS observation. To validate the findings molecular docking was conducted with lanosterol 14α-demethylase (CYP51), a significant fungal enzyme, which is the principal target of antifungal drugs. Corroboration of in vitro results with binding affinity revealed the possibility of glucopyranoside-based fatty acyl esters with stearoyl, decanoyl and lauroyl chains as highly potential compared to antifungal azole drugs.
KEYWORDS- Antimicrobial activities
- binding affinity
- docking
- fatty acid esters
- D-glucopyranoside
- gegioselectivity