JOURNAL 2407
Organic Communications
Year: 2022 Issue: 2 April-June
p.129 - 147
Viewed 2940 times.
-
Nurettin Yaylı

-
Nuran Kahriman

-
Gözde Kılıç

-
Vildan Serdaroğlu

-
Rezzan Aliyazicioglu

-
Hasan Erdinç Sellitepe

-
Sengül Alpay Karaoglu

-
Gizem Tatar Yılmaz

GRAPHICAL ABSTRACT
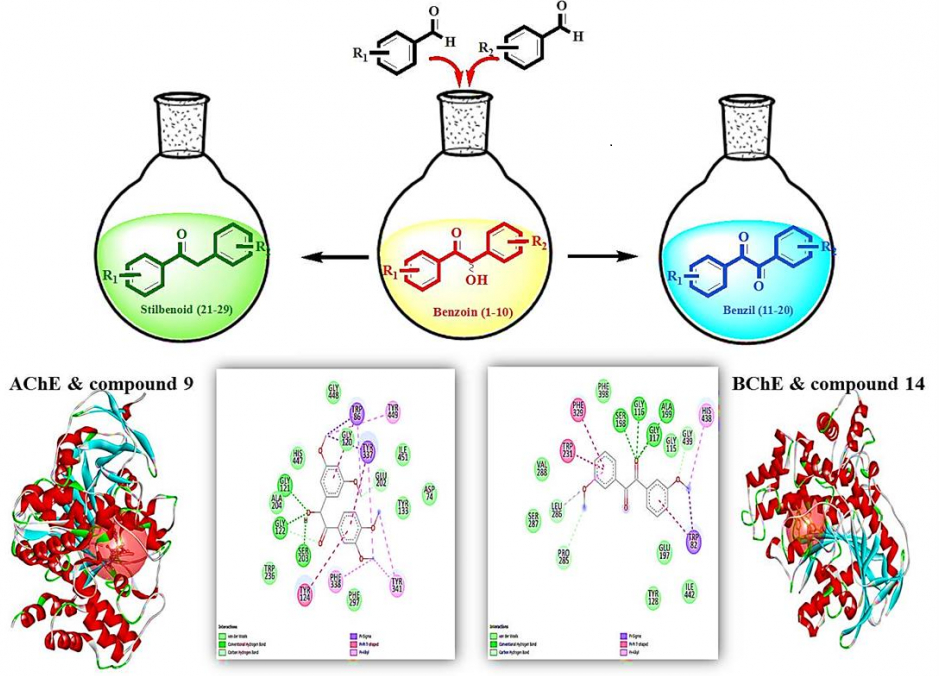
ABSTRACT
In this study, methoxy benzoin compounds (1-10) were synthesized from the corresponding aromatic aldehydes based on a screening of biological activity. Oxidation and reduction of benzoins (1-10) yielded the corresponding benzils (11-20) and stilbenoids (21-29), respectively. The enzyme inhibition, antimicrobial, and antioxidant activities of 1-29 were evaluated. 1, 14, 19, and 28 against α-amylase, 15 and 19 against α-glucosidase, 2, 4, 14, 18, 25 and 26 against tyrosinase, 2, 7, and 23 against AChE, and 7, and 13 against BChE showed similar activity to the standard used. Among the methoxy benzoin derivatives, 4 proved to be the most active compound against E.coli, Y.pseudotuberculosis, M. smegmatis, and C.albicans in the range of 41-82 µg/mL MIC values. All benzil derivatives displayed bioactivity against M.smegmatis and C. albicans. Compounds 18 and 11 were found to be most effective against M.smegmatis, and compounds 11 and 17 were found to be the most effective against C.albicans. All stilbenoid type compounds showed selective activity against B.cereus. Compounds 21 and 22 were the most effective stilbenoid compounds against M. smegmatis. Benzoins (1-10) were the most effective antioxidants among all three groups compared to the tested methods, which can be attributed to the free hydroxyl at the benzylic position. As a result, the change of carbon skeleton and substitution at different positions of synthesized organic compounds also caused the variation of biological activity.
KEYWORDS- Methoxy benzoin/benzil/stilbenoid
- molecular docking
- enzyme inhibition
- antimicrobial; antioxidant