JOURNAL 2651
Organic Communications
Year: 2022 Issue: 4 October-December
p.356 - 377
Viewed 2317 times.
-
İlker Demirbolat

-
Necla Kulabaş

-
Merve Gürboğa

-
Özlem Bingöl Özakpınar

-
Gamze Çiftçi

-
Kemal Yelekçi

-
Jianyang Liu

-
Per-Johan Jakobsson

-
Özkan Danış

-
Ayşe Ogan

-
İlkay Küçükgüzel

GRAPHICAL ABSTRACT
ABSTRACT
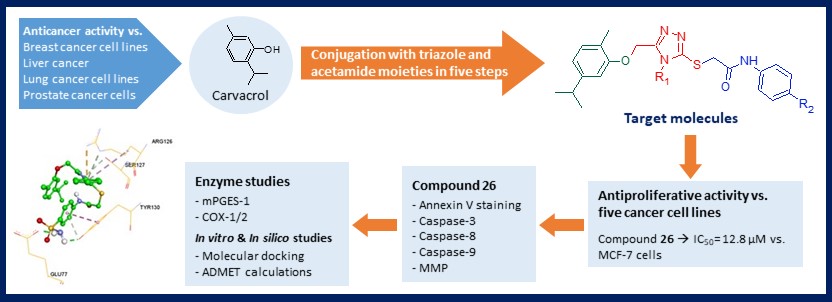
Some novel triazole-bearing acetamide derivatives 9-26 were synthesized starting from carvacrol. All synthesized compounds were characterized by FTIR, 1H-NMR, 13C-NMR, HMBC and MS data. In vitro cytotoxic activities of all synthesized molecules against five cancer lines (human breast cancer MCF-7, human lung cancer A549, human prostate cancer PC-3, human chronic myelogenous leukemia K562, human neuroblastoma SH-SY5Y cell lines) were evaluated by MTT assay. Compounds were also tested on mouse embryonic fibroblast cells (NIH/3T3) to determine selectivity. Eighteen target compounds 9-26 were screened for their mPGES-1 and COX-1/2 inhibitory activities. Of these compounds, 26 (KUC16D425) showed the highest mPGES-1 inhibition at 10 µM. This compound have also been observed to induce apoptosis and inhibit cell migration in MCF-7 cells. In silico molecular docking calculations were performed to understand the binding interactions of compounds with target proteins. ADMET predictions were also done to evaluate drug-like properties of the novel compounds.
KEYWORDS- Carvacrol
- 1,2,4-triazoles
- cancer
- apoptosis
- mPGES-1
- in silico studies