JOURNAL 2631
Organic Communications
Year: 2023 Issue: 1 January-March
p.46 - 53
Viewed 2807 times.
GRAPHICAL ABSTRACT
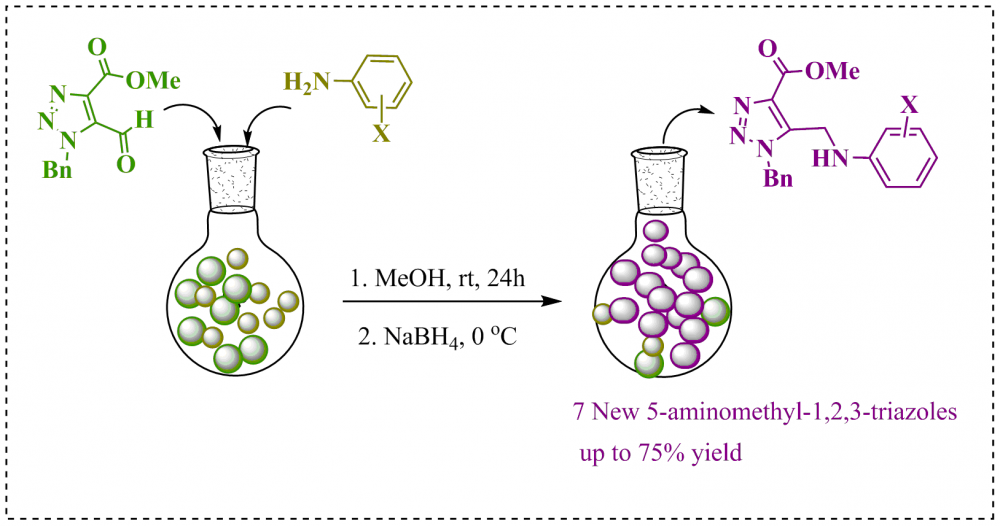
ABSTRACT
Azole motifs are commonly found in the pharmaceutical industry both as in commercial products and in the literature. In particular, the triazole motifs have many applications such as drug-like and gaining attention with the development of new anticorrosive compounds have made these motifs target molecules. In the literature, it is known that amino-triazole structures have chelating, antibacterial, enzyme inhibition, anti-TB properties, and excellent anticorrosion properties. In this study, the synthesis of novel 1-benzyl-5-(phenylamino)methyl-1,2,3-triazoles (5a-g) that could exhibit both drug-like interaction and corrosion inhibition properties was designed, synthesized and characterized. For this, 5a-g were obtained in one step from reactions of methyl 1-benzyl-5-formyl-1H-1,2,3-triazole-4-carboxylate with anilines carrying different halogen atoms in the 2 and 4 positions. The structures of all the target molecules were fully elucidated by FT-IR, 1H NMR, 13C NMR.
KEYWORDS- 1,2,3-Triazole
- 5-formyl-1,2,3-triazole
- regioselective synthesis
- 5-(phenylamino)methyl-1,2,3-triazole