JOURNAL 2742
Organic Communications
Year: 2023 Issue: 2 April-June
p.117 - 124
Viewed 3116 times.
GRAPHICAL ABSTRACT
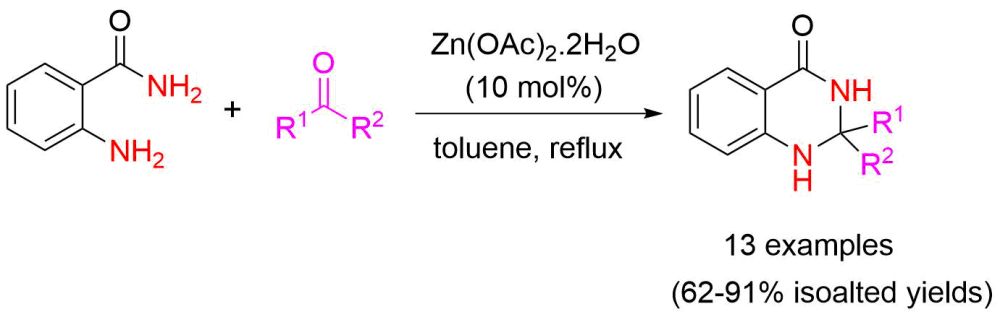
ABSTRACT
By condensation from substituted carbonyl compounds and anthranilamide under toluene reflux conditions, a wide range of 2,3-dihydroquinazolin-4(1H)-ones were produced in fair to good yields with the use of a Lewis acid catalyst Zn(OAc)2•2H2O (10 mol%), which is inexpensive, accessible, and environmentally friendly. All the synthesized compounds were properly described using melting point, IR, NMR, and mass spectral studies, and the findings were compared with information from the earlier literature. The new method has a number of advantages over the traditional methods for the synthesis of divergent 2,3-dihydroquinazolin-4(1H)-ones, including a higher product conversion, a wide substrate range, and the absence of undesirable side products. Aliphatic, heteroaromatic and aromatic carbonyl compounds were well tolerated under the optimized reaction conditions.
KEYWORDS- Quinazoline
- 2,3-dihydroquinazolin-4(1H)-ones
- catalysis
- zinc acetate dihydrate (Zn(OAc)2.2H2O)
- cyclization
- heterocycles