JOURNAL 2795
Organic Communications
Year: 2023 Issue: 2 April-June
p.98 - 107
Viewed 2695 times.
GRAPHICAL ABSTRACT
ABSTRACT
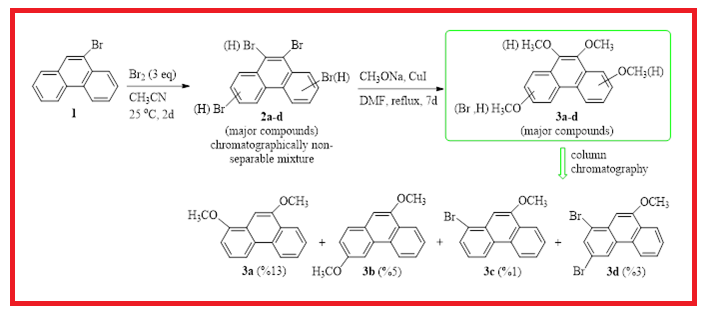
The phenanthrene bromide product mixture obtained from bromination of 9-bromophenanthrene 1, which could not be separated chromatographically, was converted to chromatographically separable methoxy products (3a-d) with CuI catalyzed methoxylation reaction. The resulting products were purified by chromatographic method and detailed structure analysis of the products was done by NMR spectroscopy. Two-dimensional NMR spectroscopy methods can be used successfully to elucidate the structures of methoxyphenanthrene derivatives. With the combination of APT, DEPT 90, HETCOR, HMBC, COSY and NOESY spectral data, the position of the substituents was easily determined. In particular, the HMBC and NOESY spectra provide critical information about the positions of the bounded groups. Further bromination reactions of 1,9-dimethoxide 3a selectively gave brominated compound 4.
- Bromophenanthrene
- methoxyphenanthrene
- bromination
- 2D NMR