JOURNAL 2887
Organic Communications
Year: 2023 Issue: 3 July-September
p.166 - 173
Viewed 2181 times.
GRAPHICAL ABSTRACT
ABSTRACT
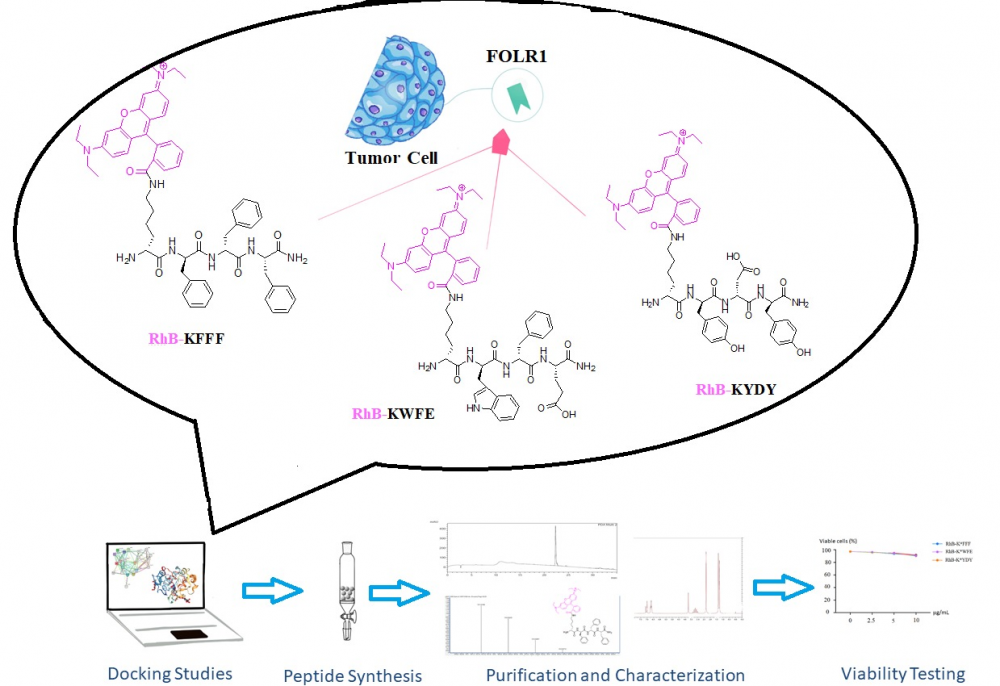
Peptides have low oral bioavailability, low plasma stability, and short circulation time; therefore, they are used in targeted strategies in cancer. In this study, according to in silico analysis, novel small peptide sequences, which are consist of three amino acid residues, with high binding capacity against the human FOLR1 surface molecule were obtained. Modeling studies were carried out to determine peptide sequences. RhB-K*FFF, RhB-K*WFE, and RhB-K*YDY peptides have been synthesized by using the Solid Phase Peptide Synthesis (SPPS) method, purified by Reverse Phase High-Performance Liquid Chromatography (RP-HPLC) and characterized by Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS/MS) and proton nuclear magnetic resonance (1H-NMR). The purity of RhB-K*FFF, RhB-K*WFE, and RhB-K*YDY peptide are 96%, 95%, and 92%, respectively. Also, cell viability test was performed for the peptides. In our further study, the peptide with highest binding affinity will be conjugated with chemotherapeutic agent in order to improve its anti-cancer activity.
KEYWORDS- Cancer
- FOLR1 protein
- molecular modeling
- peptide synthesis
SUPPORTING INFORMATION
Supporting Information
Supporting Information of "Molecular Modeling, Synthesis and Characterization of FOLR1 Specific Peptides for Tumor Targeting Activity"
Download File Supporting Information_OY_22.09.23.pdf (855.85 KB)