JOURNAL 2820
Organic Communications
Year: 2023 Issue: 3 July-September
p.152 - 165
Viewed 2702 times.
GRAPHICAL ABSTRACT
ABSTRACT
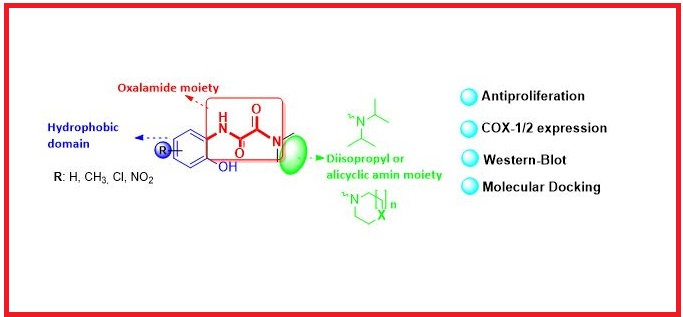
IIn the present study, new oxalamide-based compounds were designed from thalidomide and synthesized easily and with high yields (from 69% up to 93%) by a two-step method. The antiproliferative effects of synthesized 6a-d and 7a-d compounds on (ER+) MCF-7 and (ER-) MDA-MB-231 breast cancer cell line and human fibroblast WI-38 healthy cell line were investigated by the MTT method. The results showed that compound 7d was the most potent candidate against both MCF-7 and MDA-MB-231 cell lines with IC50 = 4.72 µM and 6.37 µM, respectively. To investigate whether antiproliferative effect of the compounds on breast cancer cell lines is dependent on COXs, expressions of COX-1/2 on the MCF-7 cell line were investigated by the Western-Blot technique. Among synthesized compounds, compound 7d increased the expression of both COX-1 and COX-2. The inhibition potential of compounds on COX-1/2 enzymes was investigated by molecular docking compared to inhibitor co-ligand celecoxib in crystal structures of COX-1 (PDB ID: 3KK6) and COX-2 (PDB ID: 3LN1). Docking results indeed showed that compound 7d had a higher binding affinity for both COX-1 and COX-2 active sites. Consequently, the novel oxalamide-based compounds presented here may be important candidate molecules for the development of new COX-dependent antiproliferative agents.
KEYWORDS- Oxalamides
- antiproliferation
- western-blotting
- COXs expression
- molecular docking