JOURNAL 2833
Organic Communications
Year: 2023 Issue: 3 July-September
p.125 - 133
Viewed 2297 times.
GRAPHICAL ABSTRACT
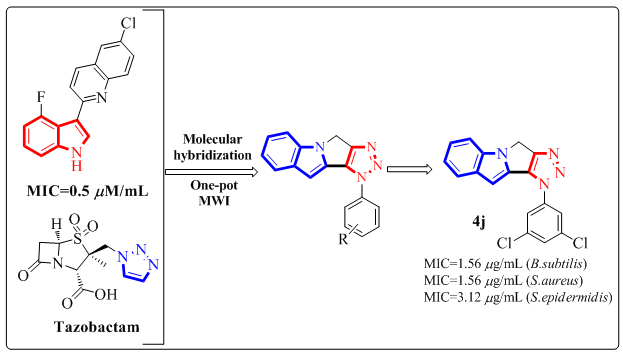
ABSTRACT
The synthesis and structural determination of several new fused [1,2,3]triazolo-[4',5':3,4]pyrrolo-[1,2-a]indole derivatives (4a-4p) utilising 1HNMR, 13CNMR, and mass spectrum analysis were discussed. The in vitro antibacterial activity of the compounds (4a-4p) against three gramme positive bacterial strains such as B.subtilis, S.aureus, and S.epidermidis revealed that the compounds 4h, 4j, and 4k demonstrated greater activity than the remaining compounds. Compounds 4d, 4i, and 4l had comparable activity to the standard. The antioxidant activity screening findings show that compounds 4c, 4d, and 4j have higher activity than conventional Trolox. Compounds 4b, 4h, 4k, and 4l have high activity, whereas the remaining compounds have moderate to low activity.
KEYWORDS- Indole
- fused 1,2,3-triazoles
- MWI
- antibacterial activity
- antioxidant activity