JOURNAL 2838
Organic Communications
VOLUME & ISSUE
Year: 2023 Issue: 3 July-September
Year: 2023 Issue: 3 July-September
PAGES
p.134 - 141
p.134 - 141
STATISTICS
Viewed 2426 times.
Viewed 2426 times.
GRAPHICAL ABSTRACT
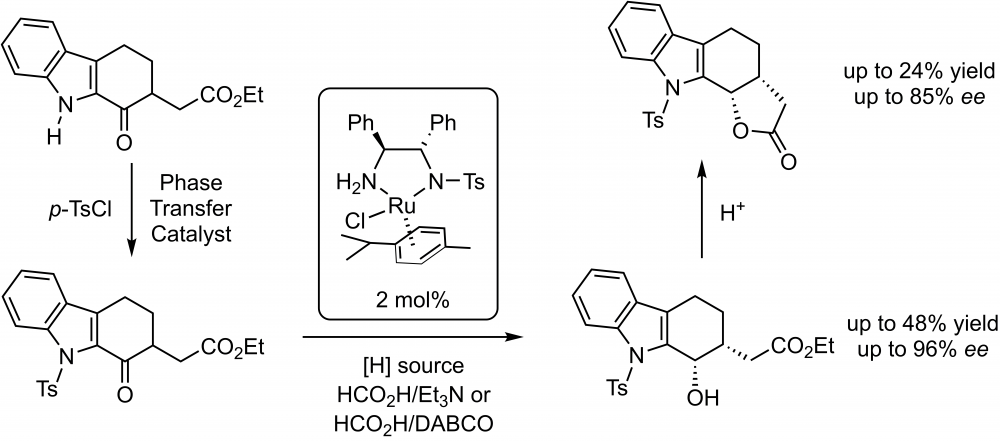
ABSTRACT
Enantioselective reduction of a new 1-oxotetrahydrocarbazole compound (ethyl 2-(1-oxo-9-tosyl-2,3,4,9-tetrahydro-1H-carbazol-2-yl)acetate) through asymmetric transfer hydrogenation by using the commercially available Noyori–Ikariya ruthenium catalyst and HCO2H/Et3N or HCO2H/DABCO as the hydrogen source have been investigated. High enantiomeric excesses (up to 96% ee) and moderate to good yields (24–72%) for corresponding alcohol and lactone compounds have been achieved. Structures of all compounds have been characterized by spectroscopic techniques.
KEYWORDS- Asymmetric transfer hydrogenation
- Noyori–Ikariya ruthenium catalyst
- Chiral tetrahydrocarbazoles
- N-Tosyltetrahydrocarbazolone
- Enantioselective lactone synthesis