JOURNAL 3376
Organic Communications
Year: 2024 Issue: 4 October-December
p.265 - 271
Viewed 1632 times.
GRAPHICAL ABSTRACT
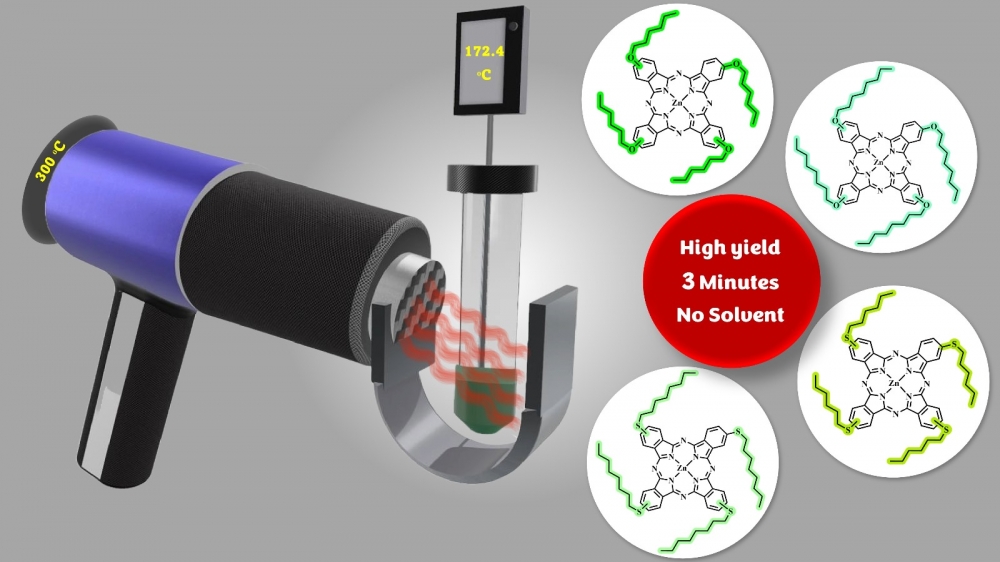
ABSTRACT
The most common method for metalated or metal-free phthalocyanines involves starting from phthalonitrile derivatives. These conventional reactions are generally carried out in high-boiling-point solvents (such as pentanol, DMF, DMSO, Quinoline, etc.) under reflux where they often have extended reaction times of up to 24 hours. New reaction methods with lower energy in short periods of time have been an issue to solve nowadays. Considering all, we aimed to synthesize substituted zinc phthalocyanine derivatives rapidly and with higher yields, without using any solvents. This study shows new synthesis methods for substituted phthalocyanine derivatives. ZnPc derivatives with different chain lengths functionalized with "O" and "S" donor atoms from the peripheral position have been synthesized in yields ranging from 52-75% in overall 3 minutes. Compared to conventional methods, which typically require extended reaction times and high energy consumption, this innovative approach reduces the reaction time from 18 hours to just 3 minutes and decreases energy consumption from 1.926 kWh to 0.063 kWh. The method achieves high reaction yields, highlighting its potential for sustainable chemical synthesis.
KEYWORDS- Solid-state synthesis
- heat-gun
- high yield
- green synthesis
- alkyl substituted phthalocyanine