JOURNAL 1248
Records of Natural Products
VOLUME & ISSUE
Year: 2019 Issue: 6 November-December
Year: 2019 Issue: 6 November-December
PAGES
p.499 - 505
p.499 - 505
STATISTICS
Viewed 3336 times.
Viewed 3336 times.
GRAPHICAL ABSTRACT
ABSTRACT
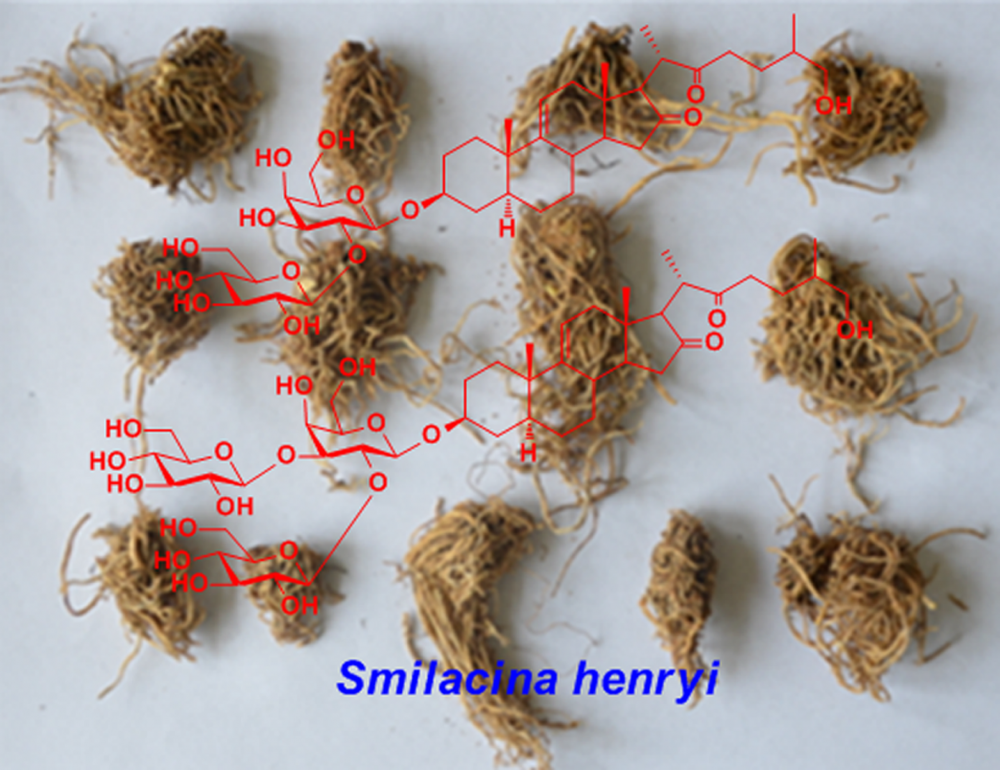
Abstract: Two new cholestanol glycosides (1 and 2) were obtained from the roots and rhizomes of Smilacina henryi. Their structures were determined as 5α-cholest-9(11)-ene-3β, 26-dihydroxy-16, 22-dione 3-O-β-D-glucopyranosyl-(1→2)-β-D-galactopyranoside (1), 5α-cholest-9(11)-ene-3β, 26-dihydroxy-16, 22-dione 3-O-β-D-glucopyranosyl-(1→2)- [β-D-glucopyranosyl-(1→3)]-β-D-galactopyranoside (2), by physicochemical properties and spectroscopic methods. In addition, the isolated glycosides were tested for their cytotoxic activity against human HepG2 tumor cells and compound 2 showed moderate activity with IC50 value of 59.32 μM.
KEYWORDS- Smilacina henryi
- cholestanol glycosides
- structure determination
- cytotoxicity