JOURNAL 1812
Records of Natural Products
Year: 2021 Issue: 4 July-August
p.281 - 292
Viewed 3101 times.
-
Danka Bukvicki

-
Miroslav Novakovic

-
Tatjana Ilic-Tomic

-
Jasmina Nikodinovic-Runic

-
Nina Todorovic

-
Milan Veljic

-
Yoshinori Asakawa

GRAPHICAL ABSTRACT
ABSTRACT
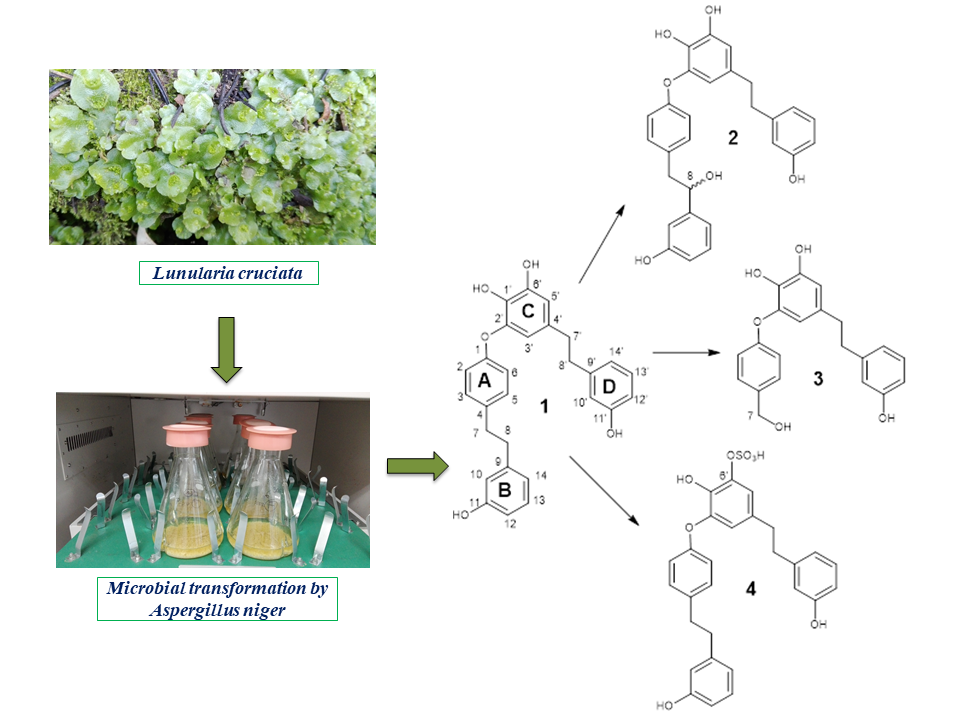
Biotransformation of bis-bibenzyl perrottetin F (1), isolated from the liverwort Lunularia cruciata by Aspergillus niger, has been investigated. New metabolites (2-4) have been isolated using reversed phase semipreparative HPLC and their structures were established to be 8-hydroxyperrottetin F, C-7-C-8 cleaved product, and perrottetin F 6’-sulfate using 1D and 2D NMR, HR-ESI-MS, IR and UV spectroscopy. The antimicrobial and cytotoxic properties of these compounds were also evaluated. Given the suggested cytotoxic properties of the parent compound, antiproliferative activity against healthy human lung fibroblasts (MRC5) and human lung carcinoma (A549) of three metabolites were evaluated revealing their lower cytotoxic properties in comparison to the starting compound - perrottetin F. The antimicrobial properties of these compounds were also evaluated, with the inhibitory activity against the Pseudomonas aeruginosa PAO1 and Staphylococcus aureus determined between 100 µM and 450 µM. The metabolites showed remarkable ability to inhibit synthesis of bacterial quorum-sensing signal molecules such as short chain acyl homoserine lactones (AHLs). Therefore, biotransformation method represents fast and effective tool for obtaining new bioactive structures.
KEYWORDS- Biotransformation;
- perrottetin F;
- liverworts;
- Aspergillus niger;
- cytotoxic activity;
- 1D and 2D NMR