JOURNAL 2000
Records of Natural Products
VOLUME & ISSUE
Year: 2022 Issue: 1 January-February
Year: 2022 Issue: 1 January-February
PAGES
p.27 - 33
p.27 - 33
STATISTICS
Viewed 2822 times.
Viewed 2822 times.
GRAPHICAL ABSTRACT
ABSTRACT
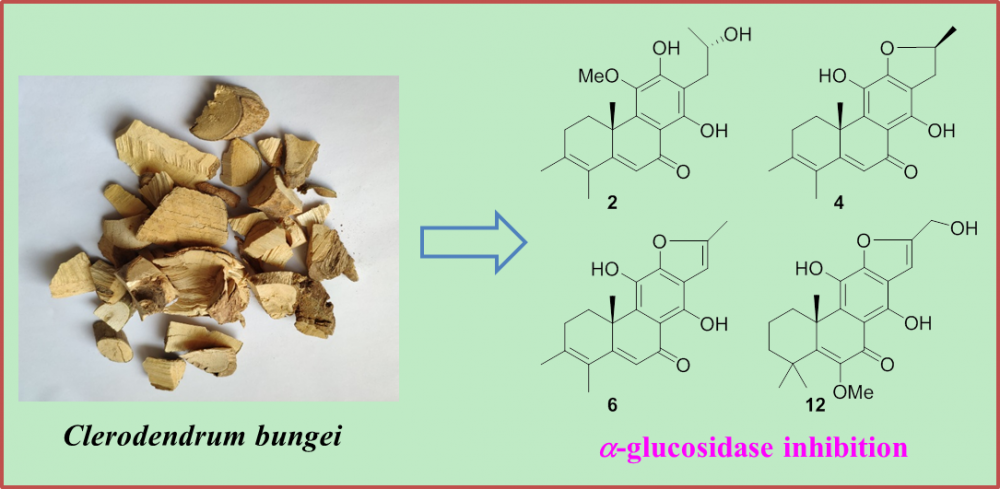
Two new rearranged abietane diterpenoids (1 and 2), together with eleven previously described analogues (3–13), were obtained from the ethanolic extract of a traditional ethnological herb, Clerodendrum bungei. The structures with absolute configurations of the new compounds were unambiguously characterized via spectroscopic methods, and that of the formerly reported crolerodendrum B (3) was corrected in the present work. Biological assessment of these isolates revealed that diterpenoids 2, 4, 6 and 12 showed significant inhibition against a-glucosidase enzyme with IC50 values in the range of 17.0–25.7 mM.
KEYWORDS- Clerodendrum bungei
- abietane diterpenoid
- alpha-glucosidase