JOURNAL 1945
Records of Natural Products
Year: 2022 Issue: 2 March-April
p.150 - 171
Viewed 2886 times.
-
Vincent O. Imieje

-
Ahmed A. Zaki

-
Ahmed M. Metwaly

-
Ibrahim H. Eissa

-
Eslam B. Elkaeed

-
Zulfiqar Ali

-
Ikhlas A. Khan

-
Abiodun Falodun

GRAPHICAL ABSTRACT
ABSTRACT
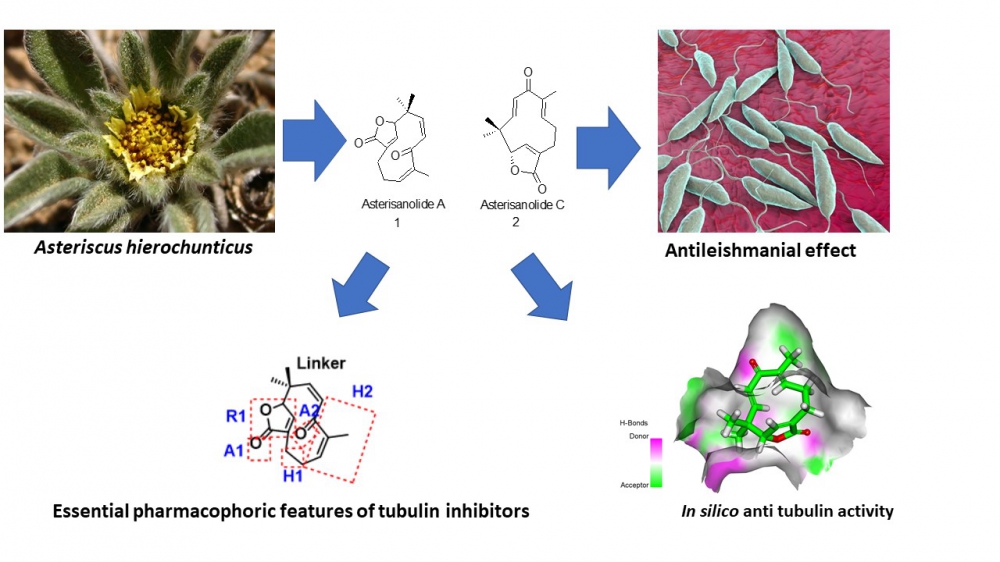
Two humulene derivatives (asteriscunolides A (1) and C (2)) were isolated from the methanolic extract of the whole plant of Asteriscus hierochunticus. Their structures were characterized by IR, 1D, and 2D NMR and HRESIMS data analysis, in addition to comparison with literature. Compounds 1 and 2 exhibited excellent in vitro antileishmanial activities against Leishmania donovani promastigotes with IC50 values of 8.38 and 3.26 µg/mL and against Leishmania donovani axenic amastigotes with IC50 values of 9.41 and 4.69 µg/mL, respectively while Pentamidine (the standard drug) exhibited IC50 values of 4.40 and 29.36, respectively. The isolated compounds possessed good activities against Plasmodium falciparum (D6) and (W2) strains with IC50 values ranging from 3.134 - 4.153 µg/mL. Furthermore, 1 and 2 showed no cytotoxic activity against the transformed human monocytic (THP1) cells. The chemical structures of 1 and 2 showed the essential pharmacophoric features of tubulin inhibitors that act through the colchicine binding site. Accordingly, molecular docking studies against the colchicine binding site of Leishmania tubulin have been preceded. Compounds 1 and 2 showed excellent binding mode with free energies of -5.62 and -5.54 kcal/mol, respectively. Further in silico studies were carried out and expected that 1 and 2 have the likeness to be drugs through exhibiting good ADMET results, no significant affinity against CYP3A4, and general low toxicity.
KEYWORDS
- Humulene derivatives
- Asteriscus hierochunticus
- antileishmanial
- antimalarial
- tubulin inhibitor