JOURNAL 2461
Records of Natural Products
VOLUME & ISSUE
Year: 2023 Issue: 2-March-April
Year: 2023 Issue: 2-March-April
PAGES
p.256 - 264
p.256 - 264
STATISTICS
Viewed 4229 times.
Viewed 4229 times.
GRAPHICAL ABSTRACT
ABSTRACT
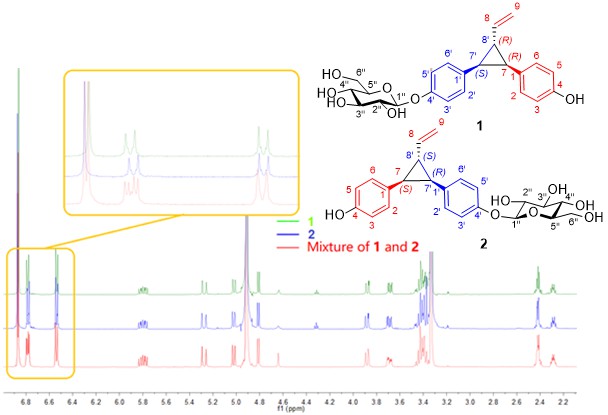
A pair of unique diastereoisomers of norlignan glycosides named cephalotanols A (1) and B (2), together with two known compounds, have been isolated from the twigs and leaves of Cephalotaxus fortunei Hook. Their structures were elucidated by using a combination of spectroscopic techniques and comparison of experimental and calculated electronic circular dichroism (ECD) data. To our knowledge, cephalotanols A and B represent the first rearranged norlignan glycosides with diphenylvinylcyclopropane core found in natural sources, of which biosynthetic pathways originating from co-occurring precursors 3S-4'-O-β-D-glucopyranosylhinokiresinol (3) and 3S-4''-O-β-D-glucopyranosylhinokiresinol (4) via di-π-methane rearrangement is proposed.
KEYWORDS- Cephalotaxus fortune
- norlignan
- diphenylvinylcyclopropane
- structural elucidation
- di-π-methane rearrangement