JOURNAL 2432
Records of Natural Products
Year: 2023 Issue: 2-March-April
p.232 - 240
Viewed 4234 times.
-
Kailing Yang

-
Tao Yang

-
Xiaolei Li

-
Ruixi Zhou

-
Rongkun Miao

-
Yuyan Guan

-
Yang Teng

-
Guanqun Zhan

-
Zengjun Guo

GRAPHICAL ABSTRACT
ABSTRACT
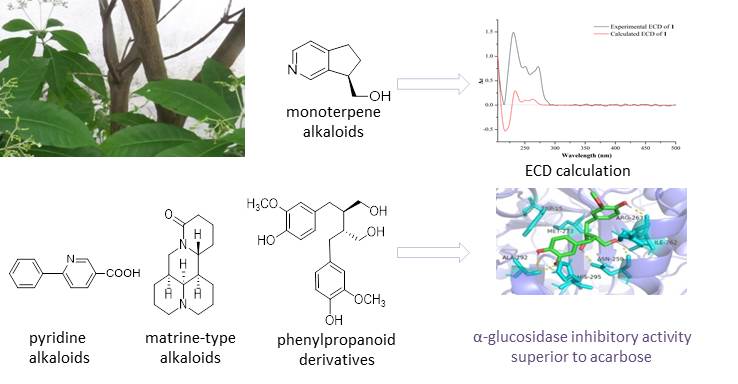
One new monoterpene alkaloid (1) and eight known compounds (2–9), belonging to two monoterpene alkaloids (1–2), one pyridine alkaloid (3), four phenylpropanoid derivatives (4–7), and two matrine-type alkaloids (8–9), were isolated from Rauvolfia vomitoria Afzel. The structure of new compound 1 was determined by extensive spectroscopic analysis and electronic circular dichroism (ECD) calculation. Notably, this is the first reported monoterpene alkaloids from R. vomitoria. Besides, compounds 3, 5, and 7–9 were first discovered in the Apocynaceae family and compounds 4 and 6 were first reported in the Rauvolfia genus. This is also the first example of matrine-type alkaloids reported in the Apocynaceae family, indicating that matrine-type alkaloids are not unique to the Leguminosae/Fabaceae family. All isolated compounds were evaluated for their anti-acetylcholinesterase, anti-α-glucosidase, and antioxidant activities. Compound 6 showed significant α-glucosidase inhibitory activity and remarkable DPPH radical scavenging capacity, both of which are superior to the positive controls. Molecular docking of compound 6 with α-glucosidase was further performed, suggesting that compound 6 could form hydrogen bonds with residues ALA-292, ASN-259, and ARG-263.
KEYWORDS- Rauvolfia vomitoria
- AChE
- DPPH
- α-glucosidase
- monoterpene alkaloids
- molecular docking