JOURNAL 3015
Records of Natural Products
VOLUME & ISSUE
Year: 2024 Issue: 2 March-April
Year: 2024 Issue: 2 March-April
PAGES
p.296 - 301
p.296 - 301
STATISTICS
Viewed 2094 times.
Viewed 2094 times.
GRAPHICAL ABSTRACT
ABSTRACT
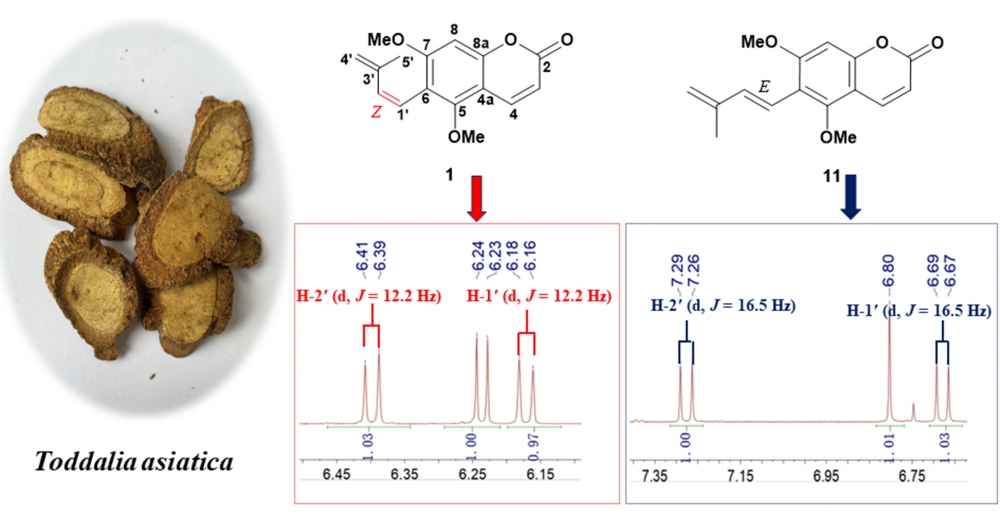
A new prenylated coumarin, 5, 7-dimethoxy-6-[(Z)-3'-methylbutan-1',3'-d-ienyl] coumarin (1), along with ten known compounds (2-11), was obtained from the roots of Toddalia asiatica (Linn.) Lam. Their structures were determined based on 1 D and 2 D NMR spectroscopy as well as high-resolution mass spectrometry. Notably, the structure of (3S, 4R)-3,4-epoxypimpinellin was revised as its cis-epimer, compounds (2-7) were isolated from this genus for the first time. The cytotoxic activities of the isolated compounds 1-11 were evaluated against SW480, A549, MDA-MB-231, HepG 2, HEp-2, SGC7901 cancer cell lines. Compound 10 exhibited weak cytotoxicity against HEP 2 with IC50 value of 75.19 ± 1.17 µM. KEYWORDS
- Toddalia asiatica
- coumarins
- chemical constituents
- cytotoxic activity