JOURNAL 3646
Records of Natural Products
Year: 2026 Issue: 1
p.9 - 9
Viewed 542 times.
-
Ali Mansour Abdul Hafid Abdelmula

-
Kaya Süer

-
Emrah Güler

-
Ahmet Özbilgin

-
İbrahim Çavuş

-
Leonie Kayser

-
İhsan Çalış

GRAPHICAL ABSTRACT
ABSTRACT
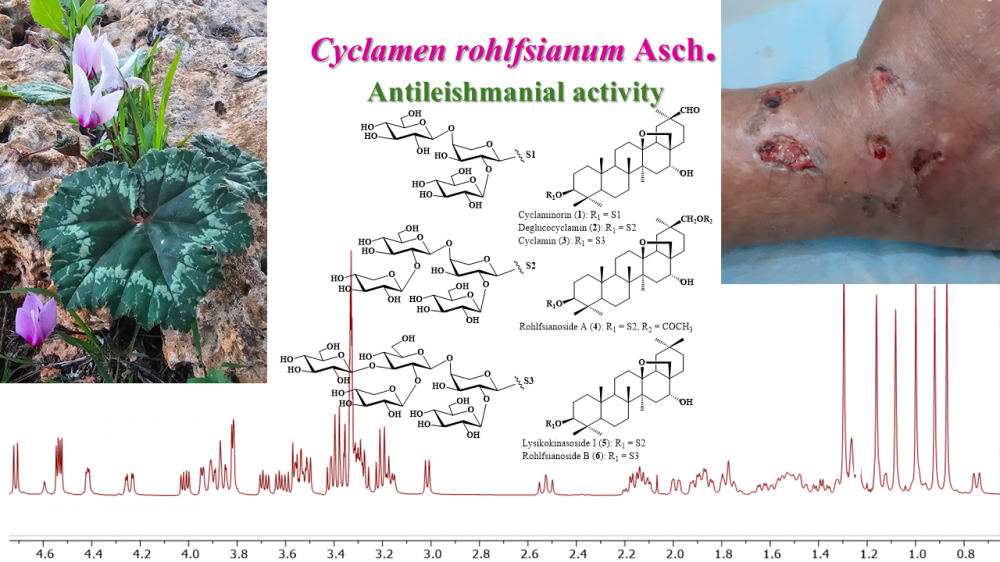
Antileishmanial activity guided studies on the methanolic extract of the tubers of Cyclamen rohlfsianum Asch. resulted in the isolation and characterization of six saponins. All saponins were established as tri-, tetra-, and pentaglycosidic derivatives of 13β-28- epoxy-oleanane-type triterpenic sapogenols. Two of them were found to be newly described saponins, rohlfsianosides A and B. Their structures were identified as 3-O-β-{[β-Dxylopyranosyl-( 1→2)-β-D-lucopyranosyl-(1→4)]-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranosyl}- 13β,28-epoxy-16α-hydroxy-30-acetoxy-oleanane (rohlfsianoside A), and 3-O- β-{{[β-D-xylopyranosyl-(1→2)]-[β-D-glucopyranosyl-(1→4)]-[β-D-glucopyranosyl-(1→4) ]}-[β-D-glucopyranosyl-(1→2)]-α-L-arabinopyranosyl}-protoprimulagenin A (rohlfsianoside B), respectively. Additionally, three known compounds, cyclamiretin A (3β,16α- dihydroxy-13β-28-epoxy-oleanan-30-al) glycosides; cyclaminorin, deglucocyclamin and cyclamen, and protoprimulagenin A (13β-28-epoxy-3β,16α-dihydroxy-oleanane) derivative, lysikoianoside I, were identified. The structure elucidation of the saponins was established by means of spectroscopic methods (1D and 2D NMR, HR-MS). All the saponins showed notable growth inhibitory activity against Leishmania ropica, with IC50 values ranging from 17.5 to 21.9/mL.
KEYWORDS- Cyclamen rohlfsianum
- 13β-28-epoxy-oleanan-type saponins
- rohlfsianosides A and B
- antileishmanial activity
- Leishmania tropica