JOURNAL 2126
Organic Communications
Year: 2021 Issue: 3 July-September
p.255 - 269
Viewed 2860 times.
-
Hasan Küçükbay

-
Fatma Müzeyyen Parladı

-
Fatümetüzzehra Küçükbay

-
Andrea Angeli

-
Gianlucca Bartolucci

-
Claudiu Trandafir Supuran

GRAPHICAL ABSTRACT
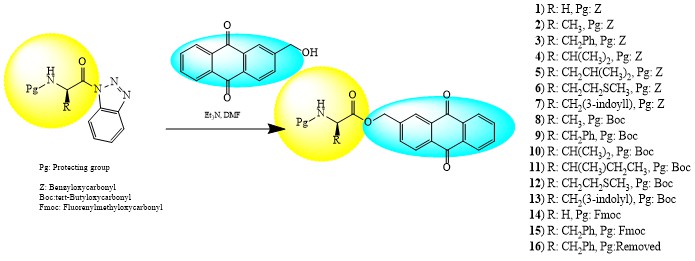
ABSTRACT
Novel monopeptide-anthraquinone conjugates (1-16) were synthesized by the reaction of appropriate N-protected amino acid with 2-hydroxymethylanthraquinone in good or high yields. The structural elucidation of the new compounds was accomplished by 1H-NMR, 13C-NMR, MS, FT-IR spectroscopy and elemental analysis techniques. The carbonic anhydrase (CA, EC 4.2.1.1) inhibitory activity of the new compounds was determined against two human (h) isoforms, hCA I and hCA II. While three of the sixteen compounds showed moderate in vitro carbonic anhydrase inhibitory properties against hCA II with inhibition constants in the micromolar level (43.5, 67.4 and 78.1 µM), they did not show inhibitory activity against hCA I up to 100 µM concentration. The antioxidant abilities of the compounds were determined using the 1,1-diphenyl-2-picrylhydrazil (DPPH) radical scavenging method, ferric ion reducing assay and metal chelation methods. While a small amount of antioxidant activity was observed according to the DPPH and ferric ion reducing power assay methods, none of the compounds showed antioxidant properties according to the metal chelating activity method at the concentrations studied.
KEYWORDS- Anthraquinone derivatives
- monopeptide anthraquinone conjugates
- carbonic anhydrase inhibition
- antioxidant activity