JOURNAL 2140
Organic Communications
Year: 2021 Issue: 3 July-September
p.270 - 279
Viewed 3546 times.
GRAPHICAL ABSTRACT
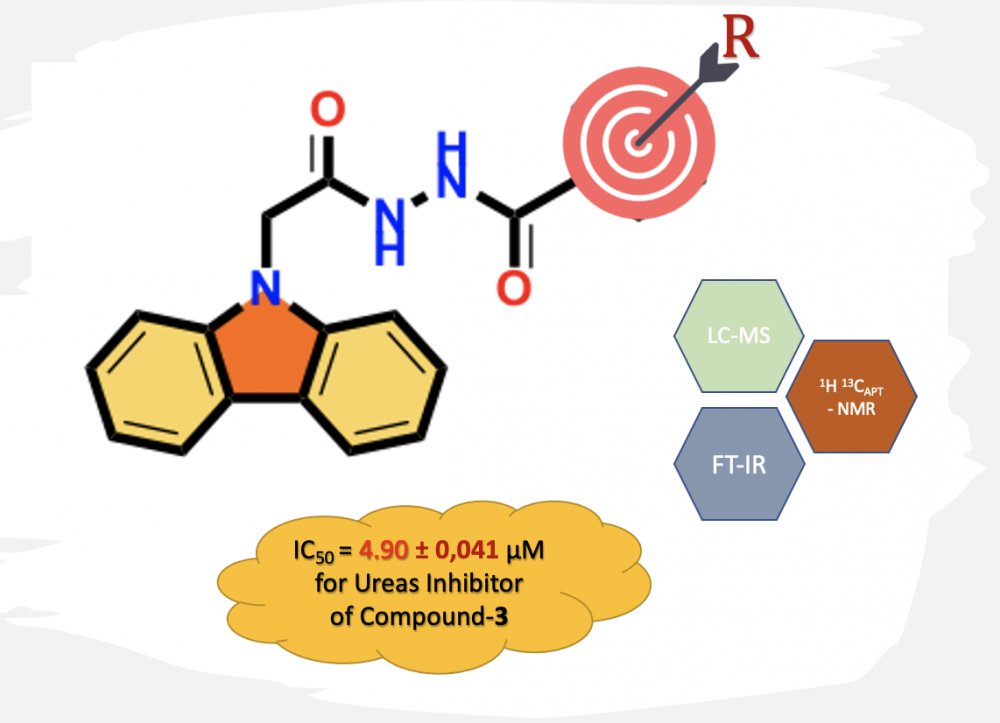
ABSTRACT
The carbazole skeleton is the key structural motif of many bioactive molecules including synthetic and natural products. The carbazole derivatives bearing different functional groups have important pharmacological activities and are widely used in medicine and pharmacology. Ethyl 9H-carbazol-9-yl acetate was synthesized from the reaction of the carbazole with ethyl bromoacetate. The acetate ester derivative was converted into the 2-(9H-carbazol-9-yl) acetohydrazide through treatment with hydrazine hydrate. The target compounds were synthesized by using carbazole-hydrazide compound and various aromatic acid chlorides. Acetyl benzohydrazide derivatives were prepared from the nucleophilic addition-elimination reactions of corresponding benzoyl chloride and 2-(9H-carbazol-9-yl) acetohydrazide in THF at room temperature condition. The structures of all newly synthesized compounds were characterized using different spectroscopic techniques, such as FT-IR, 1H NMR, 13C NMR, and HRMS. The urease enzyme activities of these compounds were investigated using Jack bean urease as the model enzyme.
KEYWORDS- Acetyl Benzohydrazide
- carbazole
- urease inhibitors