JOURNAL 2118
Organic Communications
Year: 2021 Issue: 3 July-September
p.280 - 293
Viewed 2640 times.
GRAPHICAL ABSTRACT
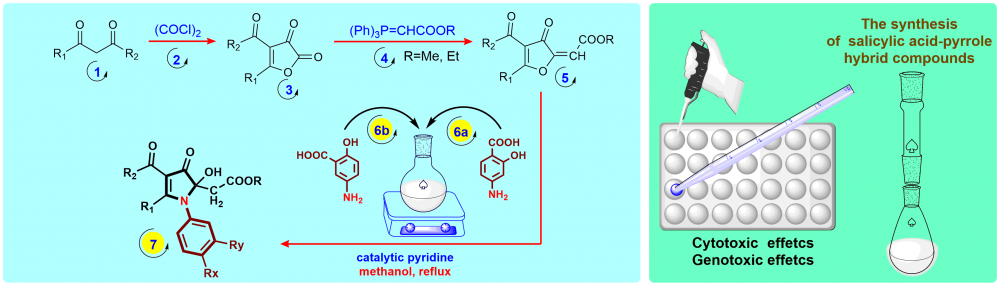
ABSTRACT
In the 20th century, many new drugs have been designed and synthesized to be used for therapeutic purposes. In these syntheses, especially the salicylic acid group is included in the structure of many drugs. The salicylic acid molecule is the starting material of aspirin and is a structurally important compound. There are many commercial products on the market that are synthesized from salicylic acid or contain salicylic acid group. In this study, the synthesis of salicylic acid-pyrrolone hybrid compounds was carried out from the reactions of furan-3-one compounds with 4-aminosalicylic acid and 5-aminosalicylic acid reagents. The synthesis reactions were achieved in three steps and in these synthesis biologically active pyrrolone and salicylic acid groups were integrated. The characterizations of these compounds that were purified by column chromatography and crystallization method were made by FTIR, NMR and HRMS techniques. The cytotoxic and genotoxic potentials of the novel compounds (7a-e) were evaluated at five different concentrations (6.25, 12.5, 25, 50 and 100 μM) using the Allium test system. As a result of cytogenetic analysis, it was determined that high concentrations of some hybrid compounds significantly reduced the number of divisions of A. cepa cells (cytotoxicity in 7a and 7c) and caused chromosomal abnormalities in dividing cells (genotoxicity in 7a, 7c, 7e, and especially 7d).
KEYWORDS- Salicylic acid;
- pyrrolone
- hybrid compound
- diastereotopic proton
- chromosome
- mitotic index