JOURNAL 2410
Organic Communications
VOLUME & ISSUE
Year: 2022 Issue: 2 April-June
Year: 2022 Issue: 2 April-June
PAGES
p.212 - 224
p.212 - 224
STATISTICS
Viewed 2257 times.
Viewed 2257 times.
GRAPHICAL ABSTRACT
ABSTRACT
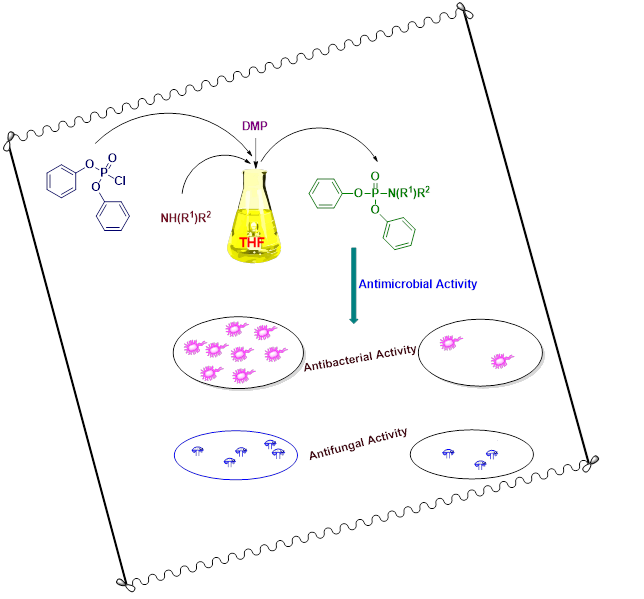
A green, facile and an efficient protocol has been used for the synthesis of new series of diphenyl substituted aryl phosphoramidates by the reaction of diphenyl phosphoryl chloride and various primary/secondary amines using THF as solvent. 1,4-dimethylpiperazine (DMP) was entrenched as a suitable base for catalysing the formation of a P−N linkage. 1H-NMR, 13C-NMR, 31P-NMR and mass spectral studies were used to characterize all the title compounds. The newly synthesized phosphoramidates were screened for their antimicrobial activity. Most of the compounds depicted good to moderate antimicrobial activity when compared to the standard.
KEYWORDS- Phosphoramidates
- P−N linkage
- 1,4-dimethylpiperazine
- antimicrobial activity
- synthesis