JOURNAL 2570
Organic Communications
Year: 2022 Issue: 4 October-December
p.324 - 332
Viewed 2791 times.
GRAPHICAL ABSTRACT
ABSTRACT
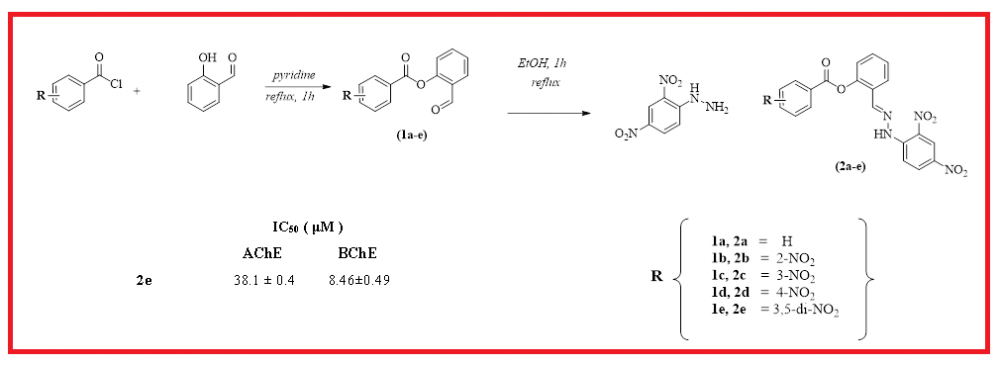
In this research, five novel hydrazone derivatives (2a-e) were obtained for the first time, characterized and investigated for their antioxidant properties, and acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) inhibitory activities. The target molecules were easily synthesized by the condensation reaction of 2,4-dinitrophenylhydrazine (2,4-DNPH) with aryl esters (1a-e) derived from salicylaldehyde as a starting material. These molecules were fully elucidated by some spectroscopic techniques and elemental analysis. Antioxidant activities of newly synthesized molecules were examined by CUPRAC reducing, ABTS and DPPH radical scavenging assays. The IC50 values of the screened molecules were determined in the range of 72.54-221.52 µM against AChE and in the range of 8.46-48.28 µM against BChE. Among the tested molecules, compound 2e indicated the highest activity against both AChE and BChE. Also, the inhibitory capacities of all tested molecules were compared to the standard molecules galanthamine. On the other hand, In CUPRAC reducing assay, the target molecules exhibited antioxidant activities in the range of 30.29 and 59.43 µM. Among these compounds, compound 2b (IC50=30.29 µM) showed the closest activity to the standard compounds butylated hydroxytoluene (BHT) (IC50=30.62 µM) and butylated hydroxyanisole (BHA) (IC50=34.24 µM).
KEYWORDS- Aryl ester
- hydrazone
- anti-cholinesterase activity
- antioxidant activity