JOURNAL 2705
Organic Communications
Year: 2023 Issue: 1 January-March
p.24 - 34
Viewed 2515 times.
GRAPHICAL ABSTRACT
ABSTRACT
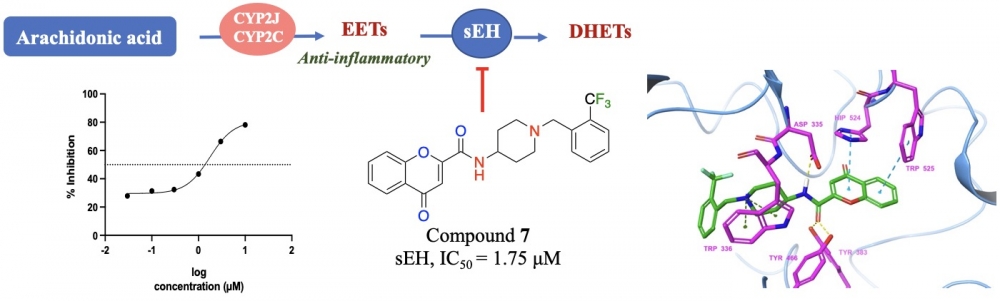
Inhibition of soluble epoxide hydrolase (sEH) is implicated as a new therapeutic approach against inflammatory disorders in the context of metabolic and cardiovascular diseases. In the course of our ongoing research on sEH inhibitors, we synthesized novel piperididine/piperazine amide derivatives of the chromone-2-carboxylic acid, and evaluated their inhibitory properties against human sEH. The chemical structures of the target compounds (2-5, 7-9) were elucidated by means of 1H-NMR, 13C-NMR and HRMS spectra. Initial screening of final compounds against sEH at a final concentration of 10 micromolar led to the identification of compound 7, which inhibited sEH activity in a concentration-dependent manner with an IC50 = 1.75 micromolar. Therefore, this chromen-2-amide derivative 7 decorated with benzyl piperidine on the amide side can be regarded as a novel lead structure, which pave the way of developing new analogues with improved inhibitory activities against sEH.
KEYWORDS- fluorometric
- inhibition
- piperazine
- piperidine
- soluble epoxide hydrolase