JOURNAL 3269
Organic Communications
Year: 2024 Issue: 3 July-September
p.144 - 165
Viewed 1894 times.
-
Sumithra Poreddy

-
Mohan Gundluru

-
Santhisudha Sarva

-
Surendra Pothuraju

-
Poojitha Bellala

-
Kranthi Kumar Konidala

-
Suneetha Yeguvapalli

-
Suresh Reddy Cirandur

GRAPHICAL ABSTRACT
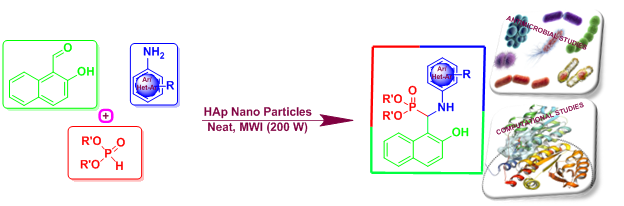
ABSTRACT
An efficient one-pot Kabachnik-Fields reaction has been carried out for the synthesis of novel α-aminophosphonates under microwave irradiation and solvent-free conditions by 2-hydroxy-1-naphthaldehyde, aromatic amines and dialkyl phosphites using Hydroxyapatite nanoparticles (HAp NPs) as catalyst. The present new protocol is environmentally benign as it offers some interesting and promising features like solvent-free, low cost, safety, minimal waste, atom efficiency, easy work-up, short reaction time and possessing excellent functional group tolerance to structurally diverse derivatives. The title compounds were subjected to antimicrobial activity against bacterial and fungal strains by disc diffusion and MIC methods. Advanced computational tools were also employed to conduct molecular docking and toxicogenomic investigations on target compounds. Employing ensemble-docking approaches, the primary contenders were identified for their potential to bind to the active sites of GlcN-6-P synthase, with PDB IDs 2VF5 and 2POC acting as target receptors. ADMET studies revealed promising drug-like characteristics of these compounds.
KEYWORDS- α-Aminophosphonates
- HAp NPs
- microwave irradiation
- solvent-free
- antimicrobial activity
- docking & ADMET studies