JOURNAL 3274
Organic Communications
Year: 2024 Issue: 3 July-September
p.166 - 177
Viewed 2058 times.
GRAPHICAL ABSTRACT
ABSTRACT
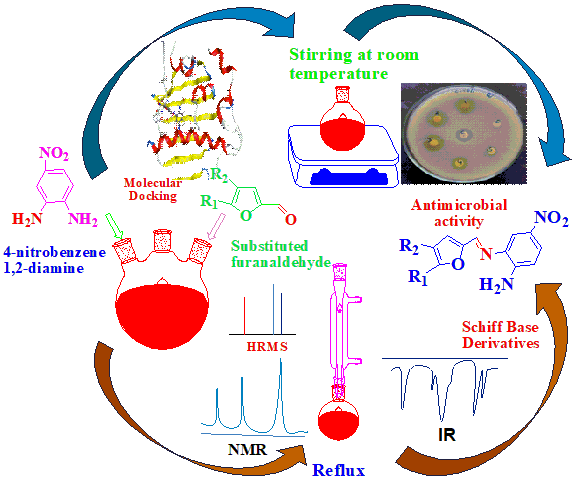
Schiff base compounds contain the azomethine group. They have diverse biological activities such as antibacterial, antifungal, antiviral, antimicrobial, and anticancer. They are frequently used as catalyst, corrosion inhibition agents and dyes in industrial process. This structure group can also be called as a main structural part of natural products and synthetic drugs. Schiff bases 3a, 3b, 3c, 3d, and 3e were synthesized by reacting 4-nitrobenzene-1,2-diamine with furfural derivatives without acid, base, or Lewis catalytic agents. Reaction progress was monitored by thin-layer chromatography. Each synthesized Schiff base was characterized by UV-visible, FTIR, 1H NMR, 13C NMR, and HRMS techniques. Nitro-substituted Schiff base 3b shows remarkable antimicrobial activities against bacterial strains E. Coli, S. aureus, and E. faecalis as compared to 3a, 3c, 3d, and 3e which was confirmed by molecular docking studies with protein of E. Coli DNA gyrase (1KZN), S. aureus cell division protein FtsZ (3VOB), and E. faecalis nucleoside diphosphate kinase(3Q8U).
KEYWORDS- Schiff bases
- synthesis
- characterization
- antimicrobial activities
- molecular docking