JOURNAL 3450
Organic Communications
Year: 2025 Issue: 1 January-March
p.28 - 37
Viewed 1604 times.
GRAPHICAL ABSTRACT
ABSTRACT
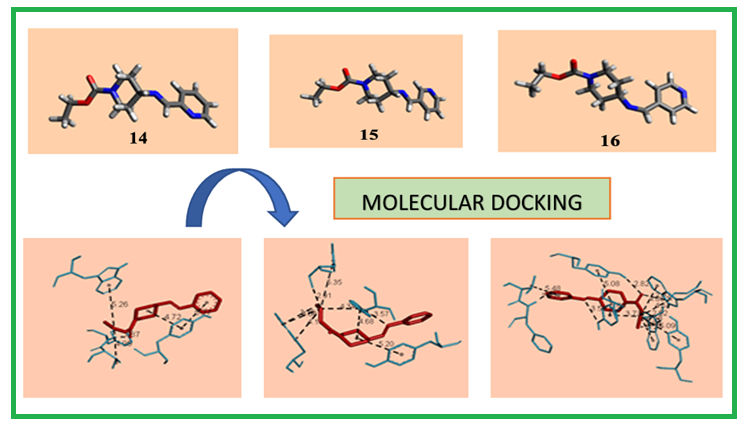
In the present study, new Schiff bases (14-16) were obtained from the condensation reaction of Ethyl 4-aminopiperidine-1-carboxylate and 2/3/4-pyridine carboxyaldehyde compounds. Desired compounds were successfully synthesized with high yields in a short time. Chemical characterization (1H-NMR, 13C-NMR and elemental analysis) and molecular docking studies of the synthesized compounds were performed against the 7XN1 structure (human acetylcholinesterase in complex with tacrine) and reference drug (Donepezil and Tacrine). It was determined that compound 16 (-7.52) had higher binding energy than Tacrine (-7.48), and compounds 14 (-7.34) and 15 (-7.41) showed values close to Tacrine (-7.48). The synthesized compounds can be potent inhibitors for hAChE.
KEYWORDS- Schiff base
- microwave
- molecular docking
- tacrine
- donepezil
- green chemistry