JOURNAL 2223
Records of Natural Products
Year: 2022 Issue: 5 September-October
p.417 - 425
Viewed 3298 times.
-
Penghua Shu

-
Yuehui Luo

-
Huiqing Zhu

-
Yamin Li

-
Yingying Fei

-
Mengzhu Yu

-
Ting Xu

-
Yueyue Lou

-
Fugang Xiao

-
Jihong Huang

GRAPHICAL ABSTRACT
ABSTRACT
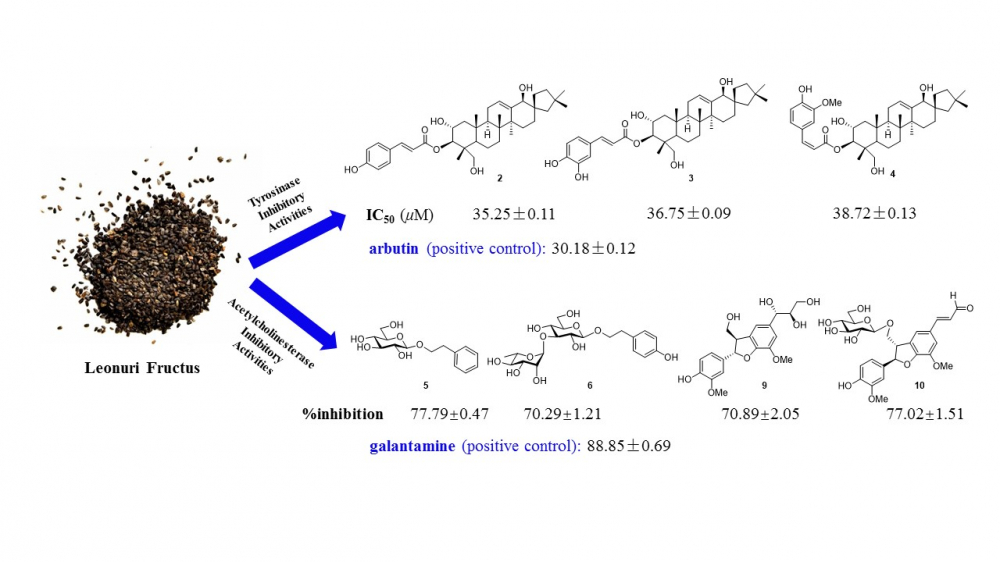
Fourteen secondary metabolites, including one cinnamate derivative (1), three spirocyclic nortriterpenoids (2–4), three phenylethanoid glycosides (5–7), four lignans (8–11) and three phenolic compounds (12–14) were isolated from the EtOH extract of Leonuri Fructus. Their structures were elucidated on the basis of 1D NMR, 2D NMR and HR-ESI-MS data analysis. All isolates were tested for their antioxidant, tyrosinase and acetylcholinesterase inhibitory activities. Most of them showed moderate antioxidant activities. Compounds 2–4 exhibited obvious inhibitory activities against mushroom tyrosinase at 25 μM, with %inhibition values of 49.36 ±2.69%, 43.43 ± 3.35%, 51.69 ± 2.81%, respectively, with arbutin used as the positive control (51.90 ± 2.57%). Compounds 3, 5–6 and 9–10 exhibited significant inhibitory activities against acetylcholinesterase, similar to the positive control, galantamine
KEYWORDS- Leonuri Fructus
- Leonurus japonicus
- nortriterpenoid
- phenylethanoid glycoside
- tyrosinase
- acetylcholinesterase