JOURNAL 2443
Records of Natural Products
VOLUME & ISSUE
Year: 2023 Issue: 1 January-February
Year: 2023 Issue: 1 January-February
PAGES
p.145 - 150
p.145 - 150
STATISTICS
Viewed 3362 times.
Viewed 3362 times.
GRAPHICAL ABSTRACT
ABSTRACT
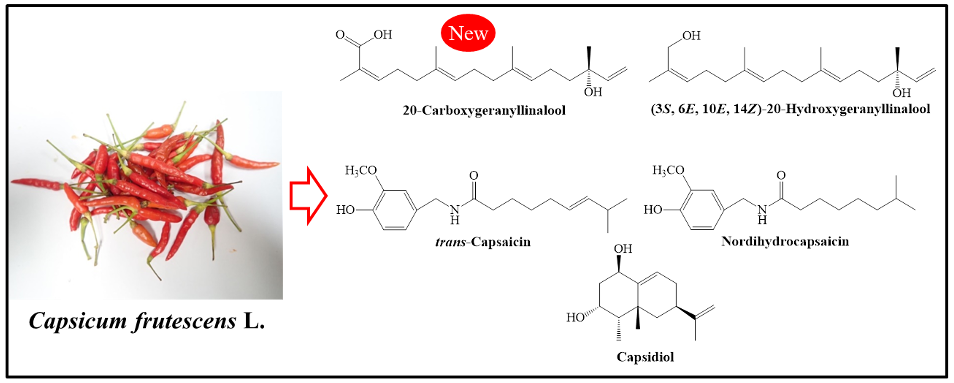
An unknown acyclic diterpene (3S, 6E, 10E, 14Z)-3-hydroxy-3,7,11,15-tetramethyl-1,6,10,14-hexadecatetraene acid (1) along with four known secondary metabolites (3S, 6E, 10E, 14Z)-20-hydroxygeranyllinalool (2), trans-capsaicin (3), nordihydrocapsaicin (4) and capsidiol (5) were isolated from the Bornean red chilli pepper Capsicum frutescens L. The structures of the secondary metabolites were determined based on spectroscopic data analysis such as NMR, HRESIMS, and IR data. The new compound 1 is a carboxylic acid precursor that would condensate with vanillylamine in the phenylpropanoid pathway in the biosynthesis of capsaicinoids. Discovery of this compound is an important milestone in our understanding of the capsaicinoids biosynthesis.
KEYWORDS- Red Chilli
- Capsicum frutescens L.
- Bornean
- acyclic
- diterpene