Bioorganic and Medicinal Chemistry Reports
Year: 2024 Volume: 7 Issue:1 January-June
1) Synthesis and antiproliferative activities against breast cancer of N-(benzimidazol-2-yl)-substituted benzamide derivatives
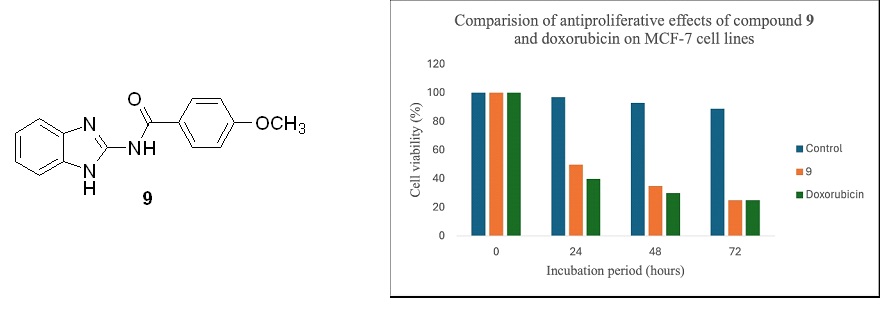
The strategic design of benzimidazole structures, akin to nucleotides, facilitates intricate interactions with amino acids within protein active sites. This structural scaffold, endowed with favorable pharmacokinetic profiles and lipophilic attributes, serves as a cornerstone in crafting potent pharmaceutical entities. In this study, a series of N-(1H-benzo[d]imidazol-2-yl)-substituted benzamides was meticulously synthesized and characterized through comprehensive spectroscopic analyses including IR, NMR, and elemental analysis. Leveraging the established pharmacophoric role of substituted benzimidazoles, renowned for their documented anti-proliferative properties, this study embarked on the synthesis of benzamides employing methoxy and substituted phenyl rings as key pharmacophores, known for their anticancer efficacy. Subsequent cytotoxicity evaluations using the MTT assay against MCF7 and normal mouse fibroblasts (L929) revealed compound 9 as the leading candidate, inducing significant cytotoxicity. This suggests its potential as a potent anticancer agent through apoptotic pathways. These findings highlight compound 9 as a promising molecular scaffold that requires careful optimization for the development of effective anticancer therapies.
DOI http://doi.org/10.25135/bmcr.32.24.03.3179 Keywords Benzimidazole benzamide synthesis antiproliferative activity MTT assay structure-activity relationship DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.2) Synthesis and antifungal activities of bisbenzazole derivatives

Invasive fungal infections (IFIs) are increasing as major infectious diseases around the world, with existing medications demonstrating limited efficacy, leading to considerable morbidity and mortality due to the absence of potent antifungal agents and the emergence of serious drug resistance. In this study, a series of bisbenzazole derivatives, featuring a methyl thio linker and either 5-nitro or chloro substituent benzimidazole ring, were synthesized using straightforward and environmentally friendly reaction conditions, and characterized via 1H NMR, 13C NMR, and IR spectral analysis. All synthesized compounds screened in vitro screening for their antifungal activity against two fungal strains, namely, C. albicans and C. parapsilosis. The compounds demonstrated significant antifungal potential, particularly against C. parapsilosis. Furthermore, molecular docking was conducted to ascertain the affinities and potential binding poses of the compounds to the catalytic regions of the target proteins 14α-demethylase (CYP51) and secreted aspartic proteases (Sapps1p). Compounds 13 and 16 exhibited the highest affinity for CYP51, with docking scores of -6.785 and -6.923 kcal/mol, respectively. The compounds' ADMET properties were assessed in silico, revealing favorable physicochemical characteristics.
DOI http://doi.org/10.25135/acg.oc.2405.32434 Keywords Bisbenzazole synthesis antifungal activity molecular docking DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.