Journal of Chemical Metrology
Year: 2024 Volume: 18 Issue:1 January-June
1) Development and application of RP–HPLC method for estimation and evaluation of stability of brinzolamide in ophthalmic formulation
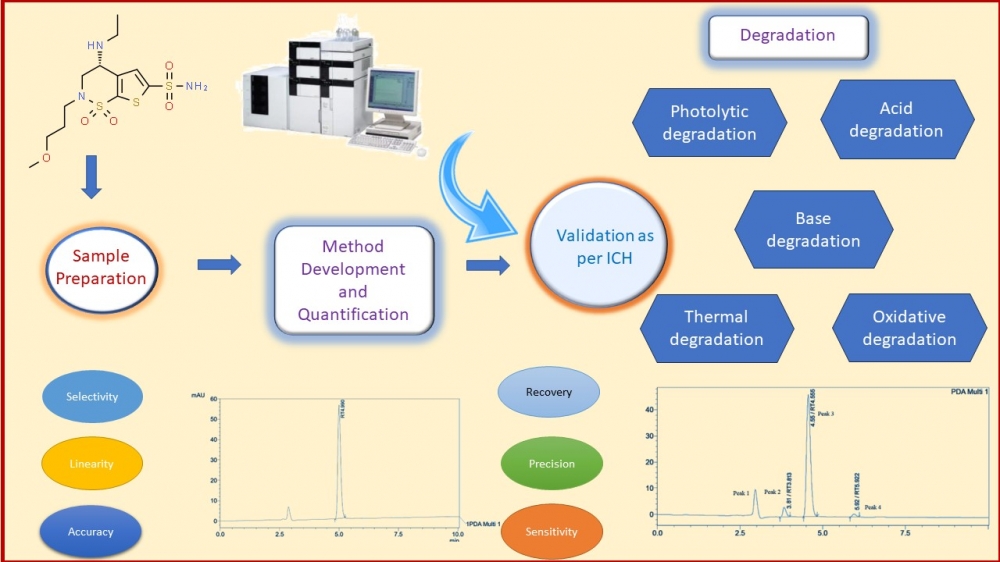
This study outlines the development, optimization, and validation of a robust reverse-phase high-performance liquid chromatography (RP-HPLC) technique for the precise quantification of Brinzolamide in ophthalmic products, aligning International Council for Harmonization (ICH) guidelines. The method employed a Phenomenex (C18) (250×4.6mm) column with 5μm particle size as the stationary phase, a 1 mL/min flow rate for the mobile phase (composed of acetonitrile: water 35:65 v/v, pH adjusted to 3 with orthophosphoric acid), and detection at 254 nm. Under these conditions, Brinzolamide displayed Rt of 4.9 minutes. The validation process, following ICH standards, exhibited excellent linearity within the 5–30 μg/mL concentration range, with a limit of detection at 0.22 μg/mL and a limit of quantification at 0.67 μg/mL. Recovery rates from ophthalmic formulations fell between 98.3%-101.08%, indicating high accuracy. Accelerated stability assessments conducted over three months revealed content retention between 98.2%-100.9%, affirming the product's stability. Additionally, Brinzolamide withstood various stress conditions without interference in quantification, as the degradation products had distinct retention times from the pure drug, offering excellent resolution. In conclusion, this RP-HPLC method is suited for routine quality control analysis of Brinzolamide in commercial ophthalmic preparations, due to its specificity, accuracy, precision, and sensitivity, aligning perfectly with ICH guidelines.
DOI http://doi.org/10.25135/jcm.2311.2949 Keywords Brinzolamide RP-HPLC method accelerated stability study force degradation degradation products DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.2) Development of an RP-HPLC method to evaluate the basic characteristics of talazoparib-loaded PLGA nanoparticles

The use of poly (ADP-ribose) polymerase (PARP) inhibitors for cancer treatment has been reported previously. Talazoparib is a PARP inhibitor, and its solubility problems encouraged us to prepare talazoparib-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles for use in brain cancer models. To determine the encapsulation efficiency and release profile, a reversed-phase high-pressure liquid chromatography (RP-HPLC) method was developed and validated. A Shiseido 5 µm C18 100 Å column (250 × 4.6 mm) was used with a flow rate of 1.0 mL/min. Isocratic elution was performed using an acetonitrile:phosphate buffer (100 mm, pH 6.25) (35:65 v/v) mixture. The injection volume was 5 μL and UV detection was performed at 227 nm. The method was linear within the range from 0.1 to 12.5 µg/mL. The encapsulation efficiency and release profile of the prepared formulation were analyzed using the developed RP-HPLC method, and it was found that the encapsulation efficiency was 65.17% ± 0.50 and the release of the talazoparib was around 40% within 5 h and remained stable for 25 h. The RP-HPLC method developed in the present study can be adapted for further applications to determine talazoparib in its commercial formulations and proposed encapsulated drug delivery systems.
DOI http://doi.org/10.25135/jcm.2310.2937 Keywords Talazoparib RP-HPLC nanoparticles drug delivery method development DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.3) Simultaneous estimation of dapagliflozin and linagliptin using reverse phase-HPLC with photo diode array (PDA)
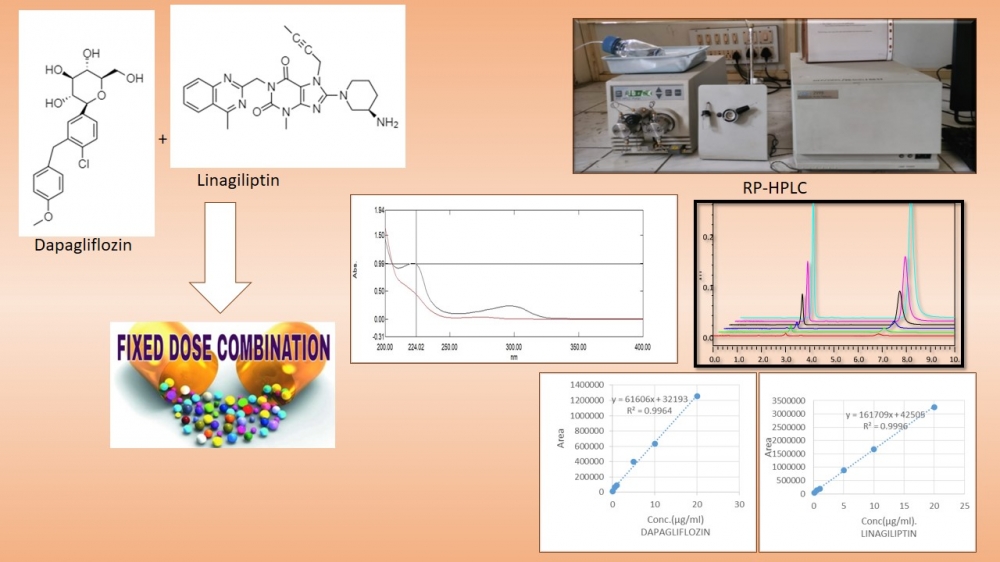
Dapagliflozin inhibits selective sodium–glucose co-transporter-2 and Linagliptin competitively and reversibly inhibits dipeptidyl peptidase-4 in fixed dose Combination (1:1) is used in the treatment of Type 2 Diabetes Mellitus. For estimation Dapagliflozin and Linagliptin in bulk and Tablet formulation, an accurate and precise method using RP-HPLC was developed and validated. In the method being discussed here was optimized using Hypersil C18 (250 × 4.6 mm, 5 µm) column as Stationary Phase, Mobile Phase being used is Acetonitrile: Water (90:10) adjusting pH 3 using Ammonium Acetate. The Flow Rate was adjusted to 1ml/min. Both Dapagliflozin and Linagliptin (1:1) sample was detected at analytical wavelength of 244nm using Photo diode array detector. The Linearity Range was between Concentration of 0.1µg/ml to 20µg/ml with correlation Coefficient of 0.995 and 0.999 for Dapagliflozin and Linagiliptin Respectively.
DOI http://doi.org/10.25135/jcm.104.2401.3020 Keywords Diabetes Mellitus dapagliflozin linagliptin RP-HPLC method validation DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.4) RP-HPLC method combined with ultrafiltration for simultaneous analysis of Melphalan and Topotecan in human plasma samples
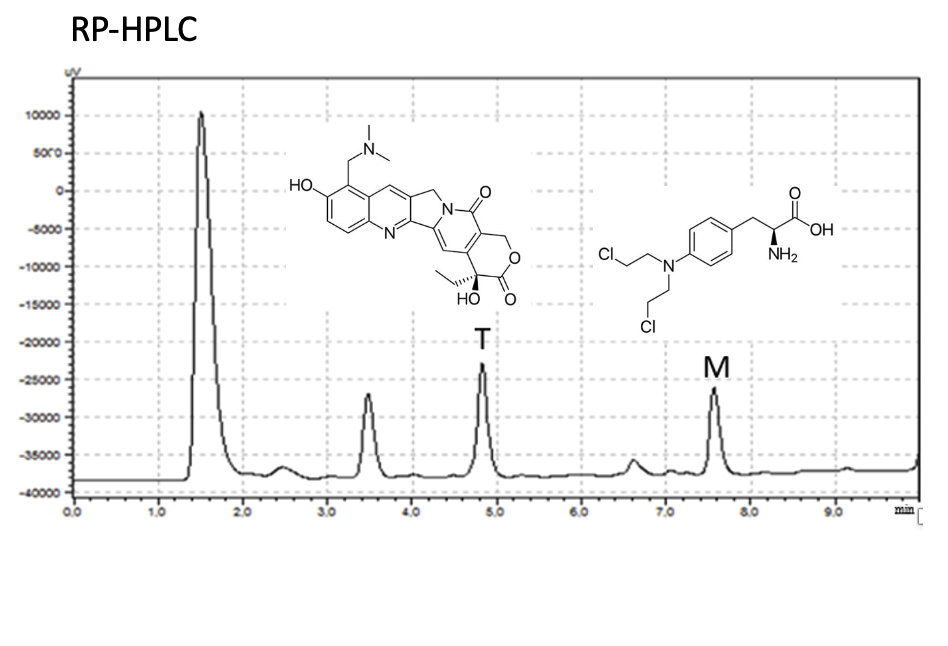
Pharmaceutical analysis still attracts the attention of researchers, and pharmaceutical analysis methods specific to the purpose of the application are needed in routine applications. In this study, the RP-HPLC method was developed for the simultaneous analysis of two different cancer drugs from human blood plasma due to simultaneous use. Melphalan and topotecan are licensed drugs that have been used for a long time. In routine practice, the simultaneous use of these two drugs is limited. However, studies have found that two active substances were used together in high-dose chemotherapy applications. This situation encourages the development of methods for the simultaneous analysis of both active substances in spiked human blood plasma samples. In this study, melphalan and topotecan were analyzed by RP-HPLC in 17 minutes, including post-run, with a gradient elution program using a Superco 5 µm C18 100 Å LC Column (100 x 4.6 mm) when the flow rate was 1.0 mL min-1. The method was linear in the 1.0- 20.0 µg/mL range for both active substances. The detection wavelength was 261 nm. The accuracy and precision of the validated analytical method were demonstrated through intraday and interday studies. The analyte was freed from the remaining plasma components due to filtering the supernatant (after precipitation of the plasma proteins with methanol) with an ultrafilter (having 10 kDa pores). The simple methodology used in this study can be easily adapted to any pre-clinical or clinical application where analysis of melphalan and topotecan in plasma is required.
DOI http://doi.org/10.25135/jcm.105.2311.2962 Keywords Melphalan Topotecan RP-HPLC pharmaceutical analysis ultrafiltration validation DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.5) Novel analytical method development and validation for simultaneous estimation of curcumin, ascorbic acid and salicylic acid in bulk and its pharmaceutical formulation by RP-HPLC
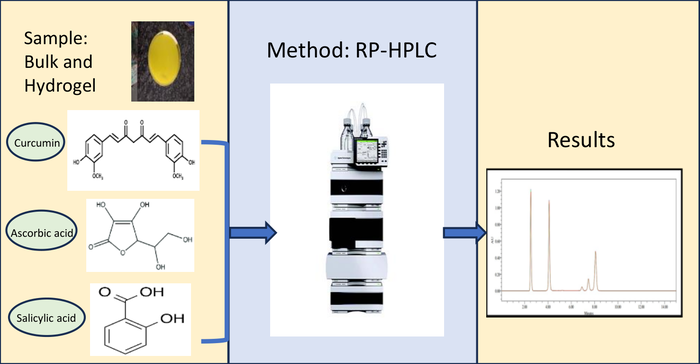
A precise and specific reverse phase high-performance liquid chromatographic method has been developed and validated to quantify curcumin, ascorbic acid, and salicylic acid in both bulk and hydrogel. Utilizing a Hypersil BDS C18 column and an isocratic mode, the mobile phase comprised of a mixture of 0.1% orthophosphoric acid and acetonitrile (50:50 v/v). The calibration range spanned concentrations of 100 - 300 µg/mL for curcumin, 50 - 150 µg/mL for ascorbic acid and 50 - 150 µg/mL for salicylic acid. The specificity of the proposed method for estimating these compounds was established through chromatographic peak purity analysis. The limit of detection and the limit of quantification were found to be 18.54 µg/mL and 56.20 µg/mL for curcumin, 10.05 µg/mL and 30.46 µg/mL for ascorbic acid, and 11.39 µg/mL and 34.51 µg/mL for salicylic acid respectively. The accuracy of the method was demonstrated by recovering curcumin, ascorbic acid, and salicylic acid from the hydrogel formulation with a recovery rate exceeding 98%. This indicates the capability of the method to accurately estimate active pharmaceutical ingredients in hydrogel dosage form without interference from excipients. Validation results support the potential applicability of the proposed method for the quantitative estimation of these three drugs in hydrogel.
DOI http://doi.org/10.25135/jcm.107.2310.2936 Keywords High Performance liquid chromatography Hydrogel Method Development Curcumin Ascorbic acid Salicylic acid DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.6) Development of liquid chromatographic (LC) method for simultaneous estimation of novel anti diabetic drug Evogliptin and Metformin
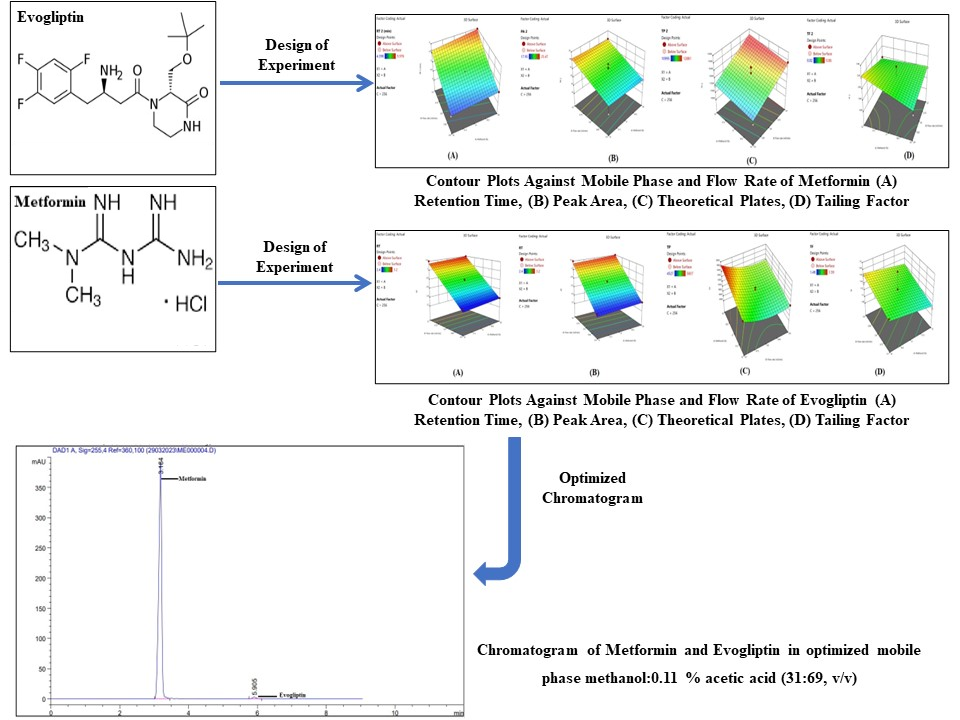
Evogliptin and Metformin are antidiabetic medications. Evogliptin works by increasing the release of insulin from the pancreas and decreasing the hormones that raise blood sugar levels. This reduces the fasting and post-meal sugar levels. Metformin works by lowering glucose production in the liver, delaying the absorption of sugar (glucose) from the intestines, and increasing the body's sensitivity to insulin. Accurate and precise high performance liquid chromatographic method has been developed for the estimation of Metformin and Evogliptin. Agilent C18 Column (250mm x 4.6mm, 5µm particle size) was used as stationary phase and methanol: 0.11% acetic acid in water (31: 69 % V/V) was used as mobile phase. The method was linear in the concentration range 50-250 µg/mL and 0.5 to 2.5 µg/mL of Metformin and Evogliptin respectively with a correlation coefficient of 0.999. The proposed method was validated with respect to linearity, accuracy, precision, and robustness as per International Council for Harmonisation of Technical Requirements of Pharmaceuticals for Human Use ICH Q2 (R2) guideline. The method was successfully applied for the analysis of Metformin and Evogliptin
DOI http://doi.org/10.25135/jcm.108.2312.2989 Keywords Metformin evogliptin design of experiment validation accuracy robustness DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.7) Trace element analysis in corn by cloud point extraction
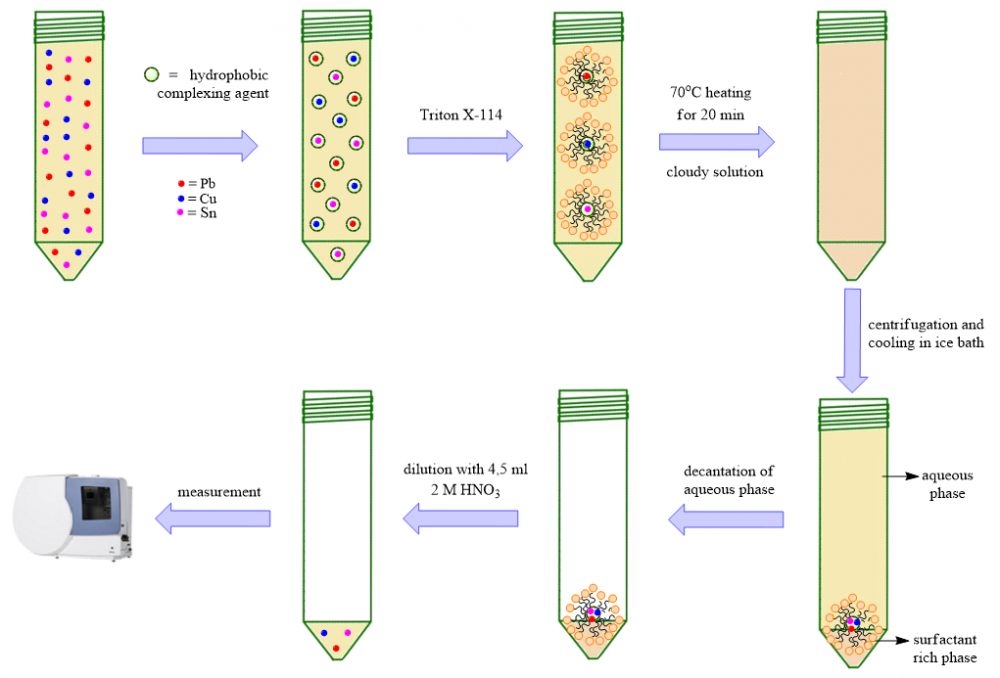
In this study, Lead (Pb), Copper (Cu), and Tin (Sn) elements determined in corn samples were analyzed from three different regions of Sakarya province. A cloud point extraction (CPE) method was used for the metal determination study and analysis by inductively coupled plasma-optical emission spectroscopy (ICP-OES). In this study, optimization processes were performed in cloud point extraction 2-(4-sulphonylamidebenzo)hydrazide-1-dithiocarbamate salt as ligand and Triton X-114 as surfactant. Three different corn samples, soil samples, and certified reference materials (GBW10011 and GBW10012) were analyzed. Recovery values in corn samples were 95.5-97.1% for Cu, 101.8-105.2% for Pb, and 94.1-97.3% for Sn; Recovery values for soil samples were 99.3-104.3% for Cu, 93.6-103.3% for Pb and 99.5-101.6% for Sn detected among them.
DOI http://doi.org/10.25135/jcm.109.2403.3180 Keywords Corn trace element 2-(4-sulphonylamidebenzo)hydrazide-1-dithiocarbamate salt ICP-OES cloud point extraction (CPE). DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.8) Determination of lead and beryllium in boron samples using 4-sulfamoylphenyl dithiocarbamate salt by ICP-OES
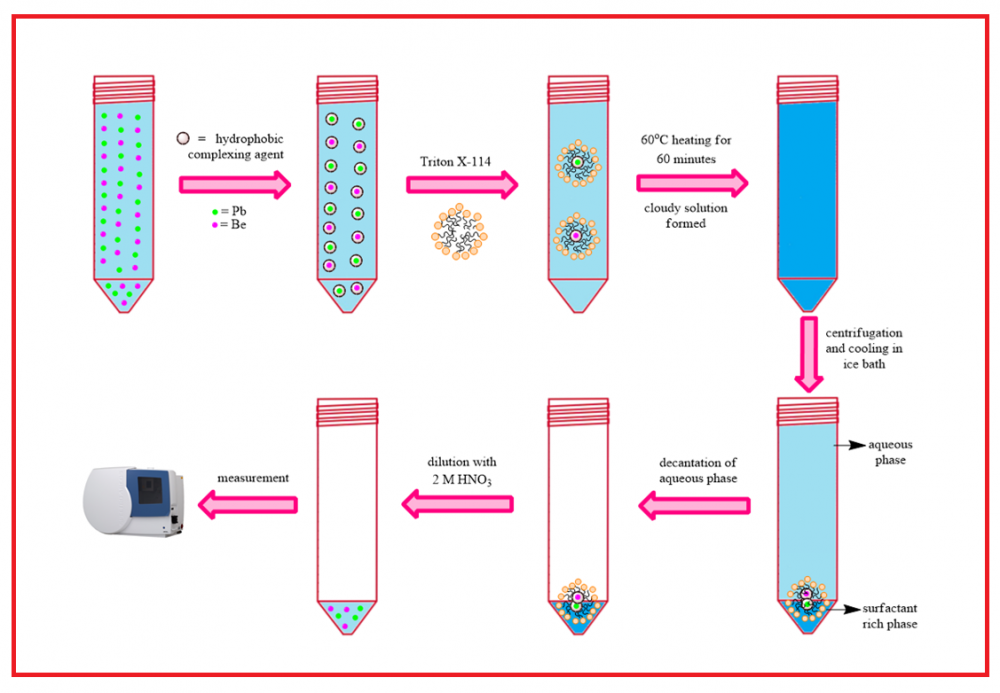
Today, high amounts of boron waste are generated due to the widespread use of boron and boron compounds. Boron waste in boron-producing enterprises in Türkiye reaches six hundred thousand tons annually. The utilization of boron wastes includes various stages, such as storage of refuse in appropriate places, recovery of boron from boron wastes, and utilization in proper sectors. In this study, the amounts of lead (Pb) and beryllium (Be) in three different boron samples (boron waste, boron ore, and processed boron ore) obtained from Kütahya-Emet region were determined by ICP-OES spectroscopic technique by cloud point extraction (CPE) method using 4-sulfamoylphenyl dithiocarbamate salt complexing agent. Colemanite was used as a boron ore in the study. According to the results, the optimum conditions for the recovery of lead and beryllium ions from aqueous media were found as pH=9, 0.12% (w/w) ligand, and 0.1% (w/w) surfactant concentration, 45 oC incubation temperature and 60 min incubation time. The RSD % of the method under the optimum conditions found were 2 and 2.5 for Pb2+ and Be2+, respectively. The procedure was successfully applied to boron waste samples, and Pb2+ and Be2+ recoveries between 96-105% were obtained.
DOI http://doi.org/10.25135/jcm.110.2404.3183 Keywords Boron waste boron ore processed boron ore 4-sulfamoylphenyl dithiocarbamate salt ICP-OES cloud point extraction DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.9) Homogeneity and short-term stability of a candidate matrix reference material from human hair for trace element measurements
Abstract: In the current project, the National Institute of Standards (NIS), Egypt, initiated a preliminary investigation to create a certified reference material for certain elements, including Fe, Mg, Mn, Al, Cu, and Zn, in human hair. This reference material would be a quality control sample for trace element determination. This paper studied homogeneity and short-term stability as key parameters for producing certified reference materials. ICP-OES and AAS were used to measure human hair powder samples, and the data was statistically evaluated for normality and outliers, which resulted in the measurement results being normal after removing the outliers. Also, sample homogeneity was evaluated and assessed using analysis of variance (ANOVA), which revealed that the human hair samples produced were homogenous and stable during transportation. This CRM mainly intends to develop methods and check instrumental performance, analytical trueness, and accuracy-related to trace element analysis in human hair and similar matrices.
DOI http://doi.org/10.251135/jcm.111.2403.3156 Keywords Biological samples reference materials homogeneity stability elemental analysis ICP-OES DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.