Organic Communications
Year: 2018 Volume: 11 Issue:4 October-December
1) Synthesis and optical properties of some isoindoline-1,3-dione compounds: Optical band gap, refractive index and absorbance band edge
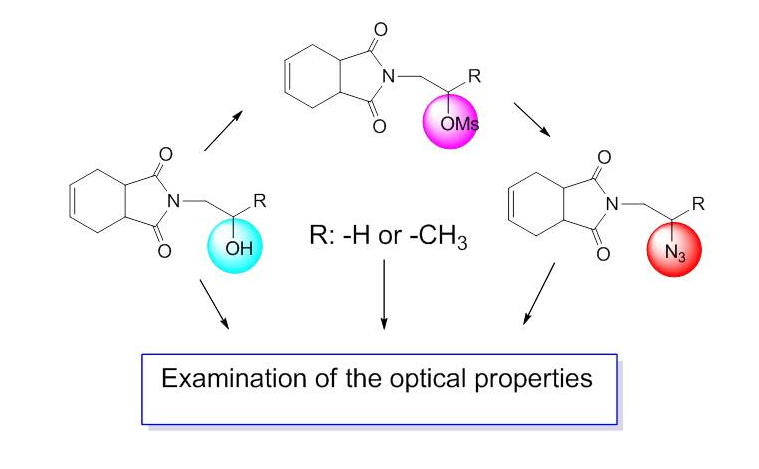
Some isoindole-1,3-dione compounds (5, 6, 7, 8, 9, and 10) have been synthesized starting from 3a,4,7,7a-tetrahydroisobenzofuran-1,3-dione (4). Compounds 5, 6, 7, 8, 9, and 10 were characterized by 1H and 13C NMR spectra, FT-IR spectroscopy, and mass spectra measurements. The optical properties of the isoindole-1,3-dione compounds (5, 6, 7, 8, 9, and 10) were also investigated. For this purpose, UV-Vis spectra of these compounds were recorded in CH2Cl2. The absorbance (Abs), transmittance (T), absorbance band edge (EAbs-be), optical band gap (Eg), and refractive index (n) of these compounds were calculated and application areas of these materials were sought.
DOI http://doi.org/10.25135/acg.oc.51.18.10.886 Keywords Isoindole-1,3-dione optical parameters refractive index dielectric materials insulator/dielectric behavior DETAILS PDF OF ARTICLE © 2018 ACG Publications. All rights reserved.2) Chemoselective reduction for different steroidal alpha, beta-unsaturated ketone into diene by using Luche reagent
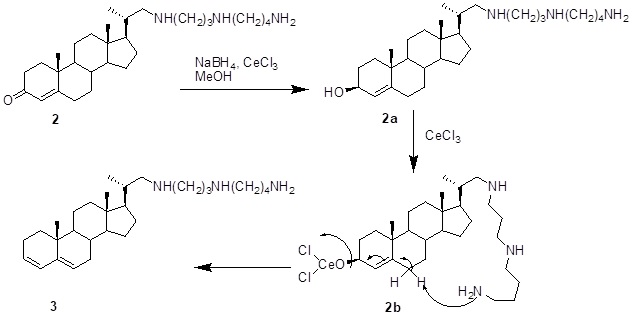
The conjugate reduction of a,b-unsaturated carbonyl compounds remains an active area of organic synthesis. Our aim is to control the reducing potential and selectivity of conjugated enone reduction. After much experimentation, the best conditions found for maximum yield with chemo and regioselectivity were to employ 1 molar eqiv. of NaBH4 for each mole of substrate in methanol containing some cerium(III) chloride. Many enones were converted essentially quantitatively to the allylic alcohol at room temperature. Surprisingly, reduction of the enone system in compound 2 (1.0 mmol) with NaBH4 (1.0mmol) and cerium chloride (1.0 mmol) in MeOH gave compound 3, in which the enone system was reduced and the allylic alcohol dehydrated producing the diene system.
DOI http://doi.org/10.25135/acg.oc.52.18.11.1039 Keywords Regioslective chemoselective reduction Luche reagent diene ketone DETAILS PDF OF ARTICLE © 2018 ACG Publications. All rights reserved.