Organic Communications
Year: 2019 Volume: 12 Issue:2 April-June
1) 3-Acyl(aroyl)coumarins as synthon in heterocyclic synthesis
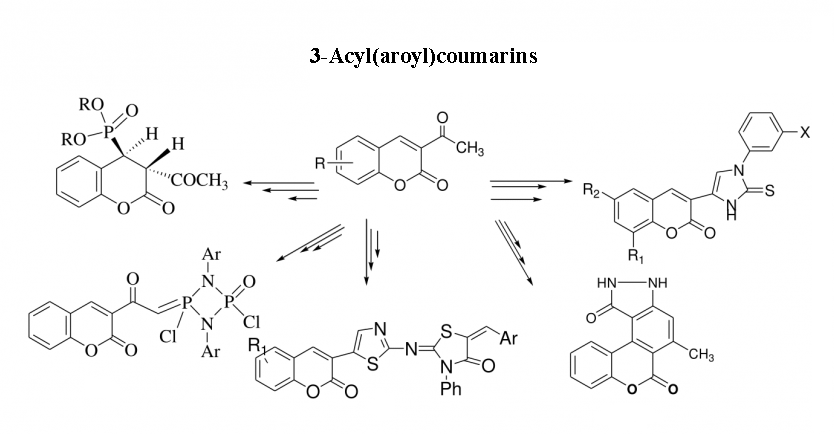
This review presents a systematic and comprehensive survey of the chemical reactivity of 3-acyl(aroyl) coumarins. The target compounds are important intermediates for the synthesis of a variety of synthetically useful and novel heterocyclic systems with different ring sizes such as isoxazole, pyrazole, 3H-triazolium salts, pyrimidine, pyridine, quinolone, benzoxocin, benzoxonin and benzoxepin.
DOI http://doi.org/10.25135/acg.oc.5619.02.1160 Keywords Coumarins Pyrazoles Thiazole Pyridine Heterocyclic Reduction DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.
2) Oxidation of hesperidin into diosmin using ionic liquids
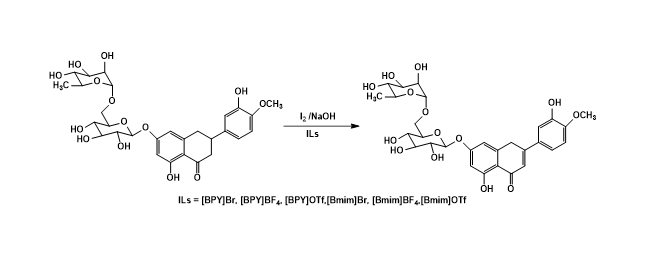
In this study, ionic liquids (ILs) were first used as solvents for conversion of hesperidin into diosmin. The diosmin was successfully synthesized by using iodine oxidation in ionic liquids including [BMIM]Br, [BMIM]BF4, [BMIM]OTf, [BPY]Br, [BPY]BF4 and [BPY]OTf. The results showed the reaction achieved in good yields (57- 76%). Among them, [BPY]Br and [BPY]BF4 exhibited as excellent solvents for the oxidation of hesperidin. In particular, water content in recovered ionic liquids (ILs), which affected strongly recyclable performance in the oxidation of hesperidin, has been investigated. It found that water content in [BPY]Br from 1 % to 5% (v/v) induced a significant increase of the synthetic yield.
DOI http://doi.org/10.25135/acg.oc.57.19.04.1242 Keywords diosmin oxidation hesperidin synthesis ionic liquid DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.3) An efficient and eco-friendly synthesis of 1, 4-dihydropyridines via Hantzsch reaction in Glycine-HCl buffer as solvent and bio-catalyst
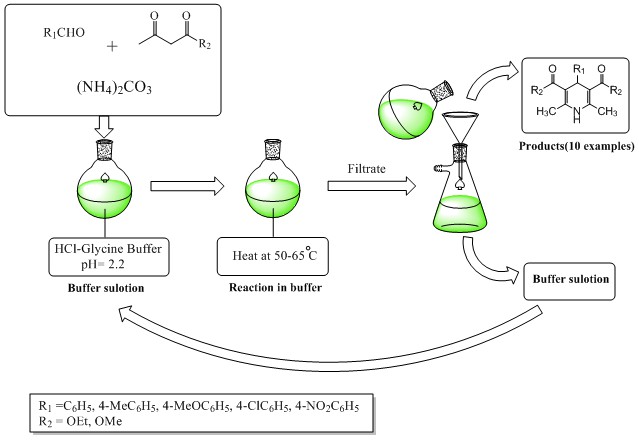
A green and efficient procedure was established to synthesize 1,4-dihydropyridines via one-pot Hantzsch reactions in buffering conditions without the use of organic solvent. In this method, a buffer solution was used as solvent and catalyst reaction; therefore, using this method has several benefits including high yields, an environmentally friendly procedure, short reaction times, and a simple work-up procedure. All the compounds were characterized by TLC, FT-IR, 1H NMR and elemental studies.
DOI http://doi.org/10.25135/acg.oc.58.19.05.1273 Keywords Hantzsch reaction 1,4-dihydropyridines glycine-HCl buffer green procedure DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.
4) The halogen effect on the ring-opening of germacyclopropylidenoids to germaallenes
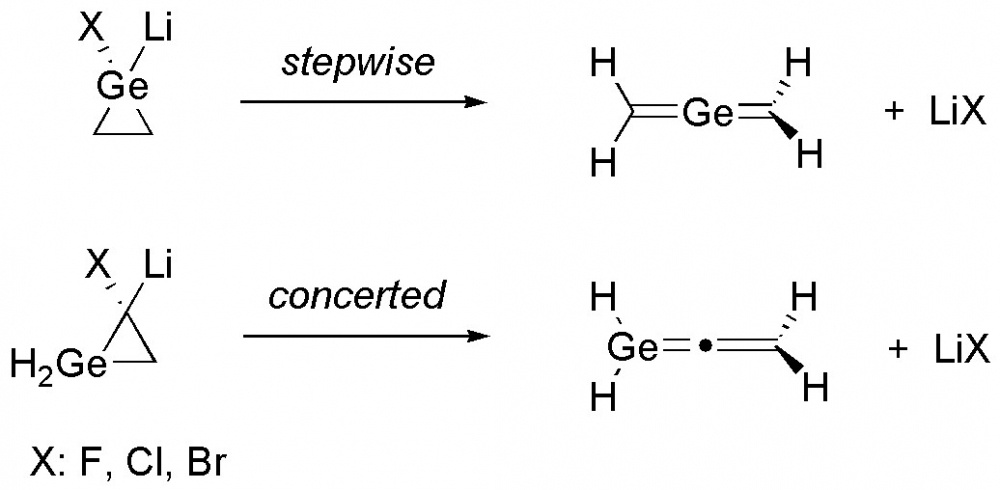
Density functional theory calculations were employed to explore the ring–opening reaction mechanisms of mono germanium analogues of cyclopropylidenoids via Doering–Moore–Skattebøl method. The theoretical findings revealed that the stepwise fashion with the intermediacy of a free germacyclopropylidene is operative for the structure with germanium atom on the carbenic position (1). Moreover, the cyclopropylidene analogue (4) of the title structures proceeds via concerted manner for the corresponding ring-opening reaction. The calculated overall energy barrier to trigger the stepwise ring-opening reaction for 1 is strongly higher than the concerted fashion for 4. Additionally, the effect of halogens (X = F, Cl, Br) on the reaction mechanism were also investigated. The energy barriers for chlorine substituted precursors are found to be lower than the fluorine and bromine substituted forms.
DOI http://doi.org/10.25135/acg.oc.59.19.05.1297 Keywords Germaallene reaction mechanism DFT germanoid germylene DETAILS PDF OF ARTICLE © 2019 ACG Publications. All rights reserved.