Organic Communications
Year: 2024 Volume: 17 Issue:1 January-March
1) Protection of aldehydes, hydroxy compounds with acetic anhydride in presence of sulfonic acid functionalized ionic liquid
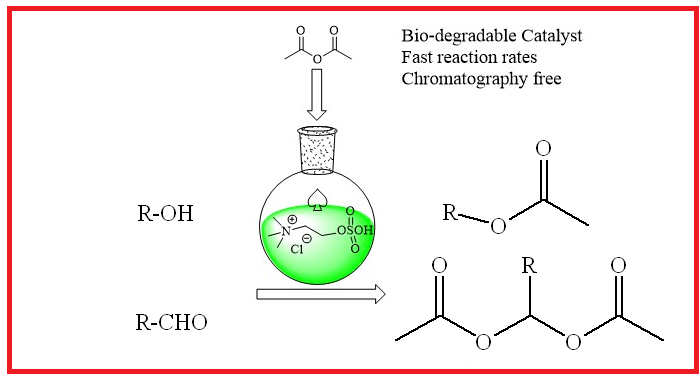
Functionalized ionic liquids (FILs) are efficient ILs for the protection of various functionalities such as aldehydes, phenol, and alcohols with acetic anhydride under neat conditions. The attracted rising interest and the subject of active research in the field of catalysis is the variety of expanded applications and yet reports on ILs are still quickly increasing. This work reports a sulfonic acid functionalized ionic liquid is an environmentally friendly, easy handling, and reusable ionic liquid catalyst for the protection of aldehydes and phenols. The catalyst was characterized with different spectroscopic techniques which indicates the fabricated IL is matching with the reported methods. The catalyst can be recycled up to eight times without substantial loss in activity. Furthermore, no chromatographic separations were needed to obtain the desired products.
DOI http://doi.org/10.25135/acg.oc.161.2312.2995 Keywords Ionic liquid acetylation room temperature short reaction time DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.2) Synthesis of ortho-carboxamidostilbene analogues and their antidiabetic activity through in vitro and in silico approaches
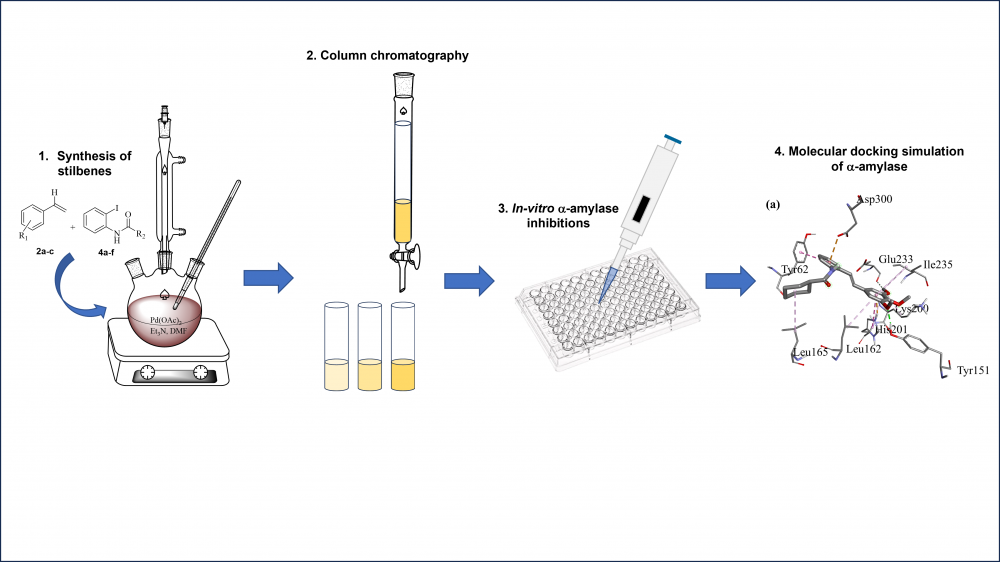
Due to the recent emergence of drug-resistance of antidiabetic drugs and increase in the number of diabetes cases around the world, the search for and discovery of more effective α-amylase inhibitors is of great interest. In the present study, a new series of eighteen ortho-carboxamidostilbene derivatives were synthesized via Heck coupling reaction. The structures of the synthesized compounds were identified by various spectroscopic techniques, including HRMS, FTIR and 1D-NMR. In addition, the compounds were evaluated in vitro for their potential α-amylase inhibitory potency using acarbose as the reference drug. Compounds 5e, 5f and 6e showed remarkably moderate to good inhibitory activity with IC50 values ranging from 13.3 − 28.2 µM. These compounds showed the potent IC50 values compared to the reference drug acarbose (IC50 = 30.2 ± 0.1). The in-silico molecular docking studies revealed that the binding interactions of the most active ortho-carboxamidostilbene derivatives (5e and 6e) with binding energies of -8.7 ± 0.0 and -8.6 ± 0.2 kcal/mol. Based on the structure-activity relationship (SAR) analysis, it was established that variations in the inhibitory activities of α-amylase enzymes were attributed to distinct types of substituents at the amide group of aryl ring A, and the number and position of methoxy groups attached to aryl ring B. These findings highlight α-amylase inhibitory properties of ortho-carboxamidostilbene-containing cyclohexyl and benzyl moieties, serving as potential lead compounds in antidiabetic drug development for the treatment of type II diabetes mellitus.
DOI http://doi.org/10.25135/acg.oc.162.2401.3039 Keywords Ortho-carboxamidostilbene Heck coupling diabetes α-amylase molecular docking DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.3) Synthesis, characterization and biological activity of novel ionic liquids with bis-imidazole moieties: antitumor, antimicrobial effects and molecular docking studies
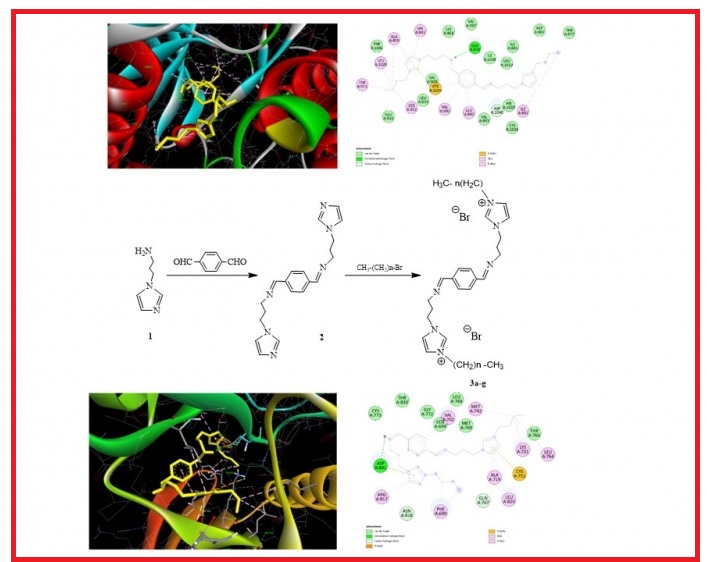
In this study, bis-Schiff bases with imidazole (2) and bis-imidazolium liquids (3a-g) were synthesized. The new compounds were characterized by Fourier transform infrared (FTIR), proton and carbon-13 nuclear magnetic resonance spectroscopy (1H- and 13C- NMR). The MTT assay was performed by our research group to measure the anticancer effect of the synthesized molecules on cell proliferation (NCI-60 screening method). Accordingly, when molecules 3e, 3g and 3f were compared with other molecules and 5-Fluorouracil (5FU) (positive control), they were found to exhibit potent anticancer activity against all cancer cell lines ((Growth inhibition (GI50): 1.0 - 1.82, total growth inhibition (TGI): 1.09 - 4.79 and lethal concentration (LC50): 2.74 – 59.5 µg/mL), but also a strong cytotoxic effect on normal lung cells Beas2B and normal cartilage cells HC (GI50: 1.0 - 1.28, TGI: 1.03 - 2.61 and LC50: 1.77 - 41.7 µg/mL). The in vitro antimicrobial activity of the synthesized compounds was evaluated against a range of microorganisms, including four Gram-positive bacteria (B. cereus, S. aureus, E. faecalis, B. subtilis,), four Gram-negative bacteria (Y. pseudotuberculosis, P. aeruginosa, E. coli, K. pneumoniae) and two yeast-like fungi (C. albicans, C. tropicalis). Molecular docking calculations were performed to investigate the ligand-protein interactions between the compounds and the Epidermal Growth Factor Receptor (EGFR) and the Vascular Endothelial Growth Factor Receptor (VEGFR1).
DOI http://doi.org/10.25135/acg.oc.163.2401.4014 Keywords Bis imidazolium ionic liquids anticancer effects antimicrobial activity molecular docking DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.4) Anticancer, antioxidant, DFT calculations, and docking studies of some new peptide-indole conjugates
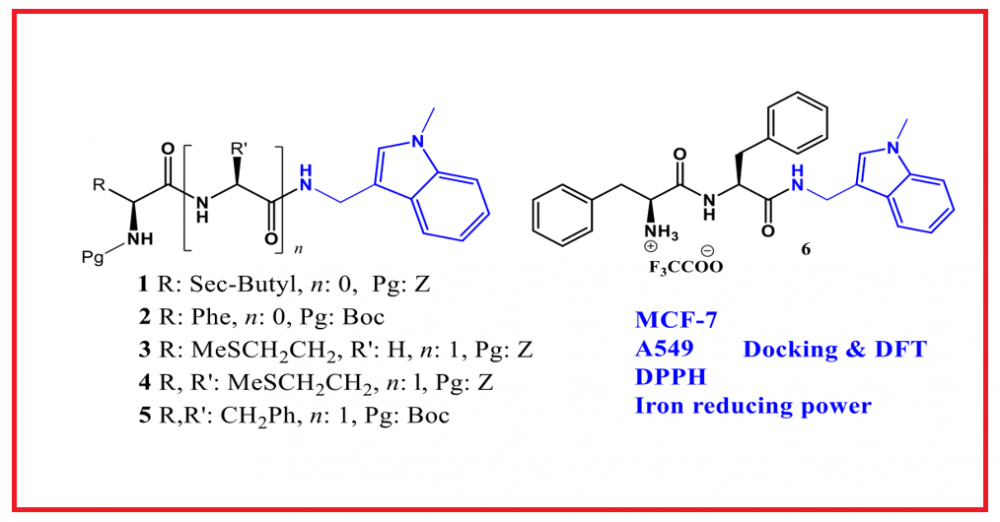
:In this study, the structures of six new peptide-indole derivatives were elucidated through spectroscopic and analytical methods following their synthesis. In addition to their anticancer and antioxidant properties, density functional theory (DFT) calculations and docking studies were conducted for the compounds. According to the obtained results, compounds 1 and 3 were identified as the most active against the MCF-7 cell line, with IC50 values of 8.72 and 5.86 μg/mL, respectively. Conversely, compounds 4 and 1 were found to be the most active against the A549 cell line, with IC50 values of 15.43 and 16.10 μg/mL, respectively. When compared to standard antioxidants using both the DPPH and iron reduction power assays, the compounds did not exhibit significant antioxidant activity. The molecular geometry and electronic properties of the synthesized peptide-indole derivatives were investigated through theoretical calculations using the Density Functional Theory (DFT) method. Molecular docking studies were also conducted to investigate the binding modes of the synthesized compounds within the active sites of EGFR enzyme.
DOI http://doi.org/10.25135/acg.oc.165.2402.3035 Keywords Indole derivatives peptides anticancer antioxidant DFT calculations docking study DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.5) Synthesis of bicyclo[4.2.0]octane ring of kingianin via [2+2] ketene cycloaddition
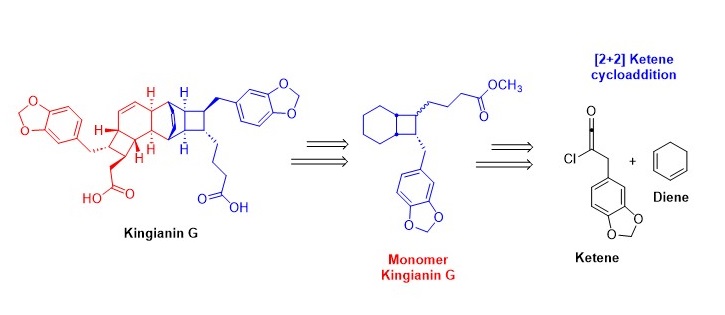
Kingianins are pentacyclic polyketides isolated from Endiandra kingiana. They exhibit promising activity in inhibiting anti-apoptotic proteins (i.e Bcl-xL and Mcl-1) and anti-diabetic proteins (α-glucosidase) at micromolar levels. Due to their structural complexity and intriguing biological activity, researchers are increasingly focussing on designing total synthesis routes for these compounds. In this communication, we propose a new non-biomimetic synthesis strategy that utilises a [2+2] ketene cycloaddition reaction as a key-step to access the bicyclo[4.2.0]octane system of kingianins. To the best of our knowledge, this is the first report based on this approach.
DOI http://doi.org/10.25135/acg.oc.164.2402.3124 Keywords Kingianins cyclic polyketides bicyclo[4.2.0]octane system [2+2] ketene cycloaddition reaction DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.