JOURNAL 2420
Organic Communications
Year: 2022 Issue: 2 April-June
p.108 - 116
Viewed 3449 times.
GRAPHICAL ABSTRACT
ABSTRACT
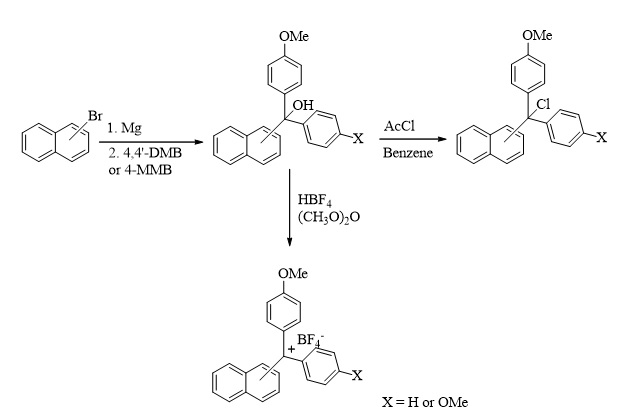
Trityl-type new protecting groups were easily prepared from the reaction of 1-bromonaphthalene or 2-bromonaphthalene and 4-methoxy substituted benzophenones following the Grignard reaction. Aryl-methyl alcohols were transferred to tetrafluoroborate salts using tetrafluoroboric acid. The alcohols also reacted with acetyl chloride to give halides of the protecting groups. The selective protection of amine, sulfur, and hydroxyl groups was obtained by the reaction of the newly synthesized protecting groups (halides and tetrafluoroborate salts). Slightly aqueous acidic conditions were used for deprotection reactions to give alcohols. It can be said that the new protective groups synthesized in this study can be a good alternative to other protecting groups in terms of low solvent use, simple procedures, economic advantages, and environmental protection.
KEYWORDS- Protecting groups
- selective protection
- kinetic
- deprotection