Organic Communications
Year: 2021 Volume: 14 Issue:2 April-June
1) Click chemistry: a fascinating method of connecting organic groups
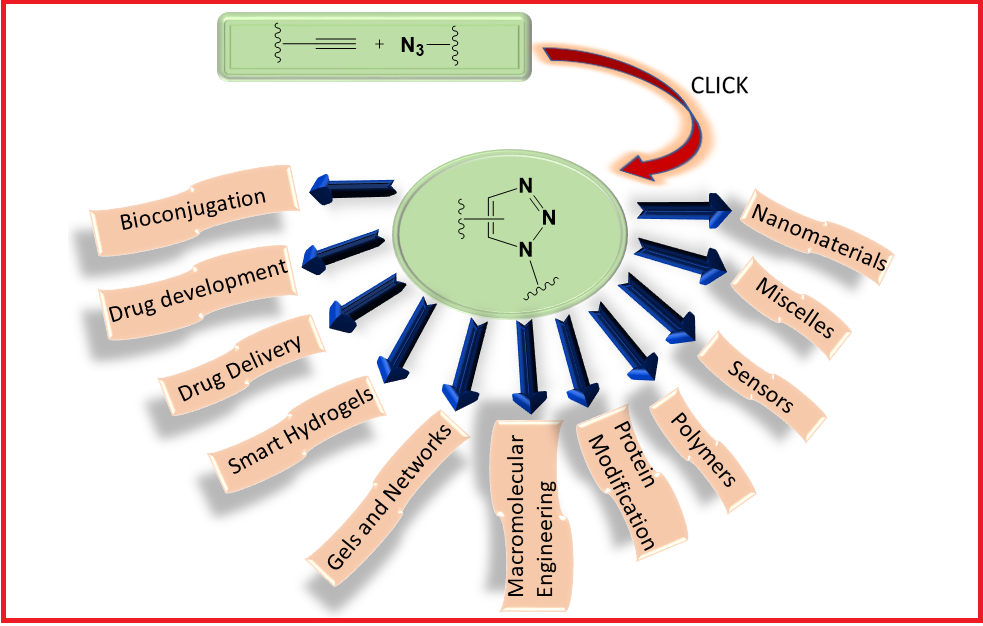
Click chemistry, a modular synthetic strategy for synthesizing the assembly of novel molecular entities, has made a tremendous impact in the field of science since its debut. This powerful strategy relies mainly upon the construction of carbon–heteroatom bonds using spring-loaded reactants. Its growing number of applications are found in nearly all areas of modern chemistry ranging from drug discovery to materials science. This manuscript includes important aspects of the copper-catalyzed Huisgen cycloaddition reaction, which is considered a gold standard of click chemistry due to its biocompatibility and reliability, along with its applications in bioconjugation, drug delivery and polymer chemistry. A bird′s eye view of recent progress in developing the copper-free click chemistry protocols such as catalyst-free strain-promoted alkyne–azide cycloaddition (SPAAC) click chemistry has also been provided.
DOI http://doi.org/10.25135/acg.oc.100.21.03.2006 Keywords Click chemistry Huisgen 1,3-dipolar cycloaddition drug delivery bioconjugation DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.2) A simple, efficient synthesis and molecular docking studies of 2-styrylchromones
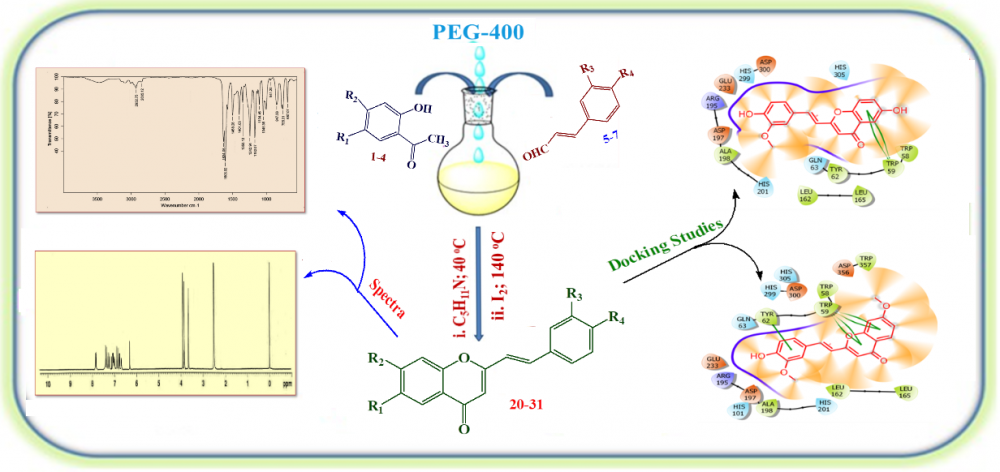
2-Styrylchromones have been synthesized successfully by using eco-friendly, recoverable and reusable PEG-400 as reaction medium in the presence of the catalytic amount of piperidine under warm conditions through 1,5-diphenylpenta-2,4-dien-1-ones as intermediates followed by oxidative cyclization with iodine in the same reaction vessel starting from 2-hydroxyacetophenones and cinnamaldehydes. The synthesized compounds were characterized by some physical methods and spectral data like IR, NMR, and LCMS etc. In silico molecular docking study was performed for all the synthesized compounds to know their ability in inhibiting pancreatic α-amylase enzyme. In this study, the compounds 24, 25, 26 & 28 with binding energies, -8.7, -8.8, -8.6 and -8.4 kcal/mol respectively were found to be more active amongst the synthesized when compared with standard drug, acarbose (-8.2 kcal/mol).
DOI http://doi.org/10.25135/acg.oc.103.21.02.1959 Keywords 2-Styrylchromones, eco-friendly PEG-400 molecular docking acarbose α-amylase DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.3) Discovery of new capsaicin and dihydrocapsaicin derivatives as histone deacetylase inhibitors and molecular docking studies
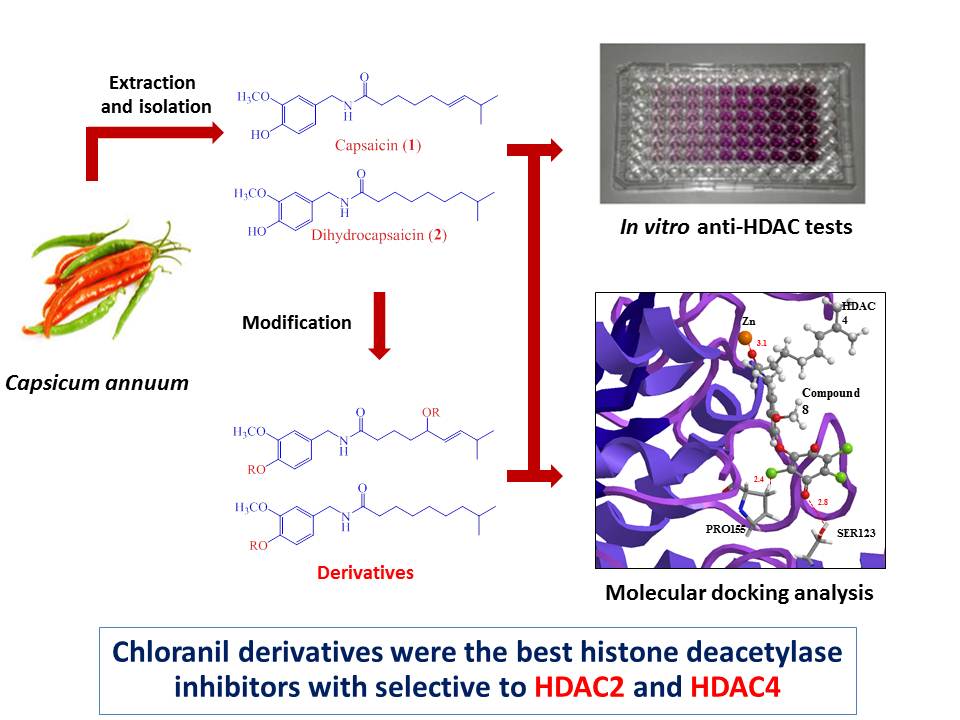
Capsaicin and dihydrocapsaicin were modified at phenolic moieties and allylic of double-bond to provide seven capsaicin and dihydrocapsaicin derivatives. Their structures were assigned by spectroscopic techniques (1D-NMR and MS). All compounds were evaluated as histone deacetylase inhibitors via in vitro fluorometric assay at 100 µM. The results revealed that the chloranil derivatives were found to be best histone deacetylase inhibitors among the tested compounds with 86% and 87% inhibitions. In addition, molecular docking experiments of the active compounds with representatives of class I (HDAC1, HDAC2, HDAC3 and HDAC8), class IIa (HDAC4 and HDAC7) and class IIb (HDAC6) HDAC isoforms displayed potential isoform-selective HDAC inhibitors. Those data show a new method for providing the isoform-selective histone deacetylase inhibitors from common natural products.
DOI http://doi.org/10.25135/acg.oc.102.21.03.1998 Keywords Red chili pepper Capsicum annuum histone deacetylase HeLa cell cytotoxiciy molecular docking DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.4) Synthesis, characterization, crystal structure and bioactivities of novel enamine and pyrrole derivatives endowed with acetylcholinesterase, α-glycosidase and human carbonic anhydrase inhibition effects
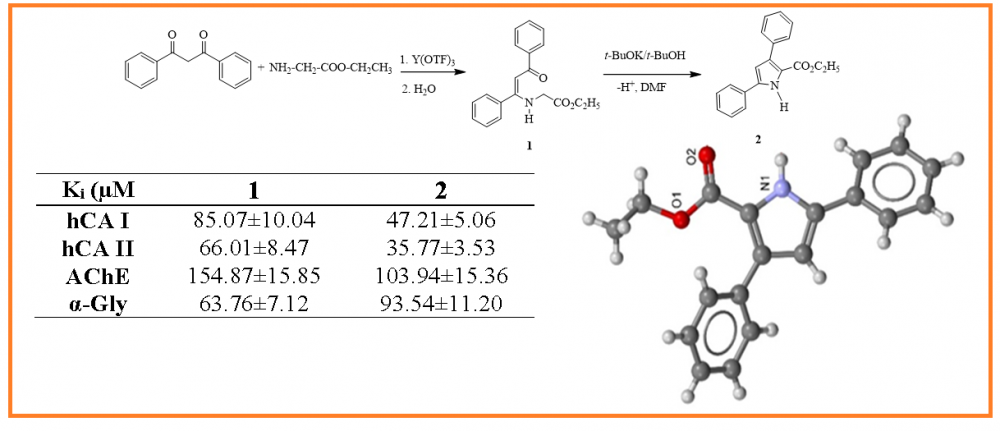
Presented research work is devoted to the synthesis of new heterocyclic compounds containing the ethyl ester fragment of acetate and glycine and the study of their crystal structure and biological activity. (Z)-Ethyl 2-(3-oxo-1,3-diphenylprop-1-enylamino)acetate (1) was first obtained on the base of the reaction of dibenzene methane with glycine ethyl ester hydrochloride in the presence of Y(OTF)3 catalyst in aqueous medium. At the same time, ethyl-3,5-diphenyl-1H-pyrrole-2-carboxylate (2) was synthesized from the interaction of enamine with tert-BuOK in the presence of tert-BuOH/DMFA solvent. The structure of new compounds has been studied by 1H, 13C NMR. In addition, the crystal structure of ethyl-3,5-diphenyl-1H-pyrrole-2-carboxylate (2) is presented. The monoclinic, yellow crystals, with sizes 0.20 × 0.10 × 0.10 mm3, one striped: a = 10.5340(6) Е, b = 7.5101(5) Е, c = 20.2352(15) Å, β = 102.131(2)°, V = 1565.09(18) Е3, space group P21/c, Z= 4, ds = 1.236 mg/m3, μ = 0.080 mm-1 were obtained. The crystalline compound keeps crystallographically independent molecules in the central bicyclic moiety. Compound 2 holds complex three-organic compound system consisting of pyrrole and benzol rings. In this study, the IC50 and Ki values of the compounds were calculated to compare their inhibition profiles on acetylcholinesterase (AChE), α-glycosidase and hCA I, and II isozymes. These compounds demonstrated Ki values in the low micromolar range for studied enzymes. The best inhibitor for hCA I and II isoenzymes and AChE was the (1) with Ki values of 47.21±5.06, 35.77±3.53 and 103.94±15.36 µM, respectively. On the other hand, compound 2 showed the best inhibition profile against α-glycosidase with Ki of 63.76±7.12 µM.
DOI http://doi.org/10.25135/acg.oc101.21.04.2029 Keywords Enamine acetylcholinesterase pyrrole carbonic anhydrase α-glycosidase DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.5) The use of boron carbide powder as an efficient green catalyst for Friedel-Crafts chemistry
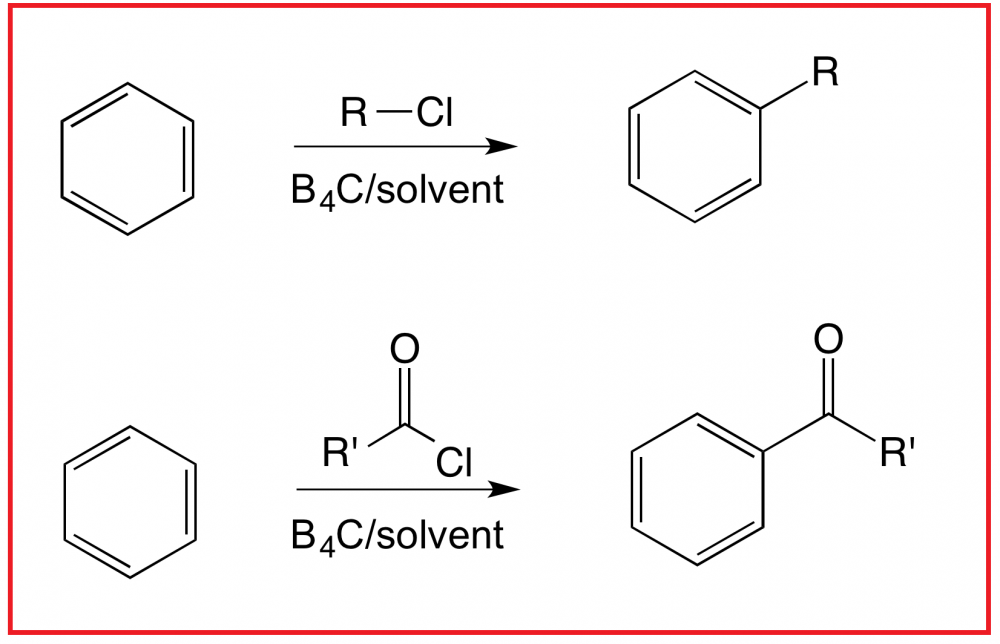
Boron carbide (B4C) as finely powdered solid functions has been found to function as an efficient, green, non-toxic, and easily recoverable heterogeneous catalyst system for the Friedel-Crafts alkylation and acylation reaction.
DOI http://doi.org/10.25135/acg.oc.104.21.04.2023 Keywords Friedel-Crafts acylation Friedel-Crafts alkylation Boron carbide catalyst Green chemistry DETAILS PDF OF ARTICLE © 2021 ACG Publications. All rights reserved.