Records of Natural Products
Year: 2026 Volume: 20 Issue:2
1) Comprehensive phytochemical profiling and cell-free in vitro bioactivity assessment of the endemic Campanula kirikkaleensis Dönmez & Güner

This study presents the first comprehensive evaluation of the phytochemical profile and cell-free antioxidant and enzyme inhibitory activities of the endemic Campanula kirikkaleensis Dönmez & Güner. n-Hexane, chloroform, and 70% methanol extracts from aerial parts and roots were investigated for their phytochemical composition and bioactivities. GC-FID/MS analyses revealed phytol (14.2%) as the main volatile in aerial parts and hexadecanoic acid (22.5%) in roots. MSD-SPME identified 3-ethyl-3,4-dihydro-2(1H)-quinoxalinone as the major shared volatile (9.8% in aerial parts; 14.5% in roots). Linoleic acid was the predominant fatty acid in both parts (26.4% in aerial parts; 22.3% in roots). LC-HRMS highlighted rutin (525.29 mg/100g dry plant) in aerial parts and chlorogenic acid (24.78 mg/100g dry plant) in roots as dominant phenolics. The 70% methanol aerial extract showed the strongest antioxidant activity (DPPH IC₅₀: 0.14 ± 0.02 mg/mL; CUPRAC: 375.1 ± 12.2 mM Trolox), while the highest TEAC value was recorded in the chloroform root extract (1.27 ± 0.02 mM). The same methanol extract also demonstrated the most potent α-amylase inhibition (IC₅₀: 0.537 ± 0.042 mg/mL). ICP-OES analysis revealed calcium as most abundant in aerial parts (24.9 mg/g) and potassium in roots and flowers (11.7 and 19.0 mg/g). These findings provide a detailed chemical and bioactivity profile of C. kirikkaleensis, supporting its potential for future pharmacological investigations.
DOI http://doi.org/10.25135/rnp.2509.3644 Keywords Campanula kirikkaleensis phytochemical profile antioxidant activity LC-HRMS GC-FID/MS. DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.2) GC-MS profiling and in vitro assessment of antioxidant and 5-lipoxygenase inhibitory activities of essential oils from five indigenous Hedychium species in Thailand
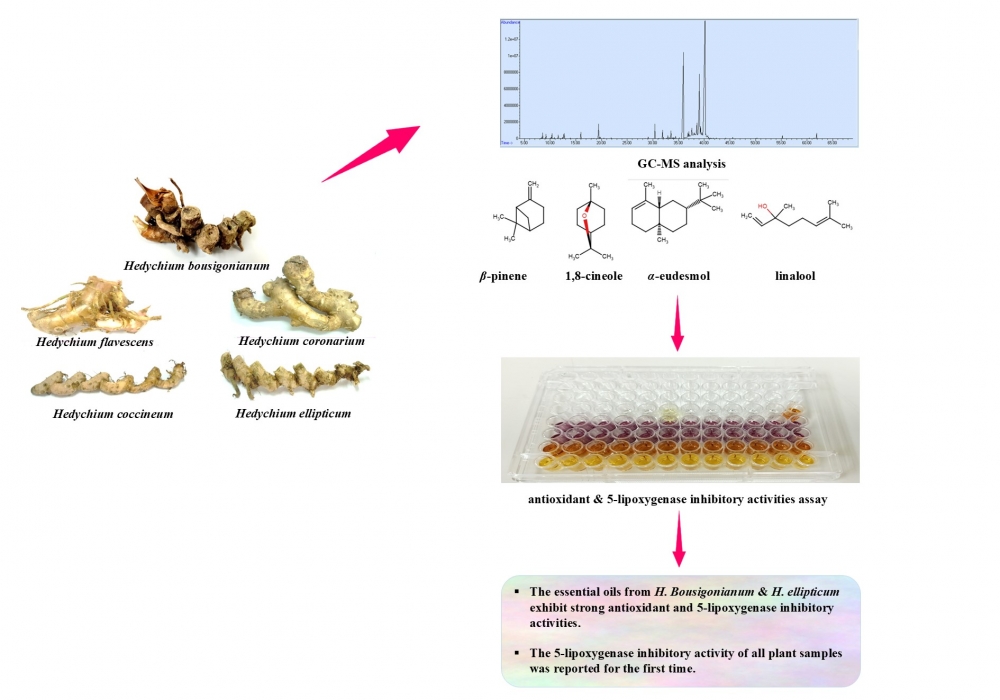
The genus Hedychium (Zingiberaceae) consists of rhizomatous aromatic plants widely utilized in Thai traditional medicine and cosmetic formulations. Although recognized ethnopharmacological, the phytochemistry and bioactivity of Thai Hedychium species have great potential for drug development. This study investigated the chemical profiles, in vitro antioxidant capacities, and in vittro 5-lipoxygenase (5-LOX) inhibitory activities of essential oils hydrodistilled from the fresh rhizomes of five Thai species—H. bousigonianum, H. coccineum, H. coronarium, H. ellipticum, and H. flavescens. Gas chromatography–mass spectrometry (GC–MS) analysis revealed distinct, species-specific chemotypes, characterized by α-eudesmol (37.41%) in H. bousigonianum; β-pinene (27.42% and 35.38%, respectively) in H. coccineum and H. flavescens; linalool (26.40%) in H. coronarium; and 1,8-cineole (89.36%) in H. ellipticum. The essential oils from the fresh rhizome of H. bousigonianum, H. coccineum, H. coronarium, H. ellipticum, and H. flavescens demonstrated antioxidant activity across multiple assays. Their free radical scavenging capacity was confirmed by the DPPH assay (IC₅₀ = 17.92 ± 0.62, 20.26 ± 0.53, 20.02 ± 0.89, 18.27 ± 0.60, and 22.12 ± 0.60 µg/mL, respectively) and the ABTS assay (IC₅₀ = 13.16 ± 0.95, 19.02 ± 0.43, 18.09 ± 0.34, 11.81 ± 0.67, and 19.34 ± 0.27 µg/mL, respectively). Similar trends were observed in the superoxide anion (IC₅₀ = 28.41 ± 0.64, 28.96 ± 0.51, 31.47 ± 0.58, 26.19 ± 0.61, and 29.12 ± 0.42 µg/mL, respectively) and hydroxyl radical assays (IC₅₀ = 26.67 ± 0.73, 27.98 ± 0.37, 28.92 ± 0.31, 25.34 ± 0.81, and 32.45 ± 0.55 µg/mL, respectively). Furthermore, the essential oils showed 5-LOX inhibitory activity (IC₅₀ = 57.32 ± 1.09, 67.50 ± 1.70, 60.31 ± 1.25, 55.84 ± 1.53, and 75.34 ± 2.28 µg/mL, respectively), supporting their potential anti-inflammatory properties. Notably, the essential oils from H. bousigonianum and
H. ellipticum showed the most potent dual antioxidant and anti-inflammatory activities, highlighting their promise as natural sources of bioactive compounds for future pharmaceutical development.
3) Two undescribed phenylpropanoid derivatives from Notopterygium incisum roots with anti-inflammatory potential
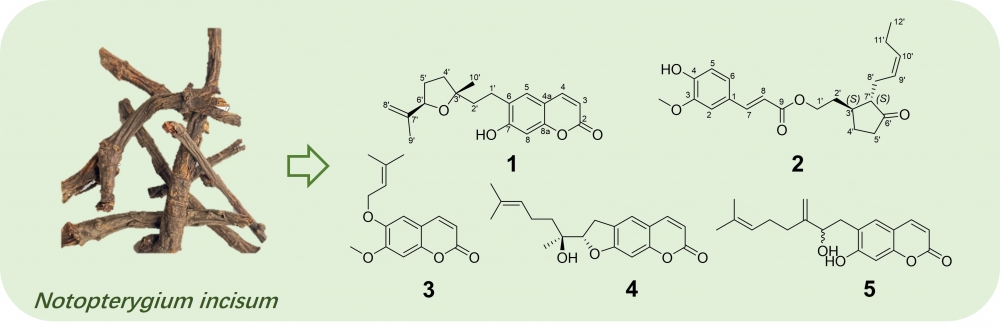
Two previously undescribed phenylpropanoid derivatives, including a terpenylated coumarin (1) and a ferulic acid analogue (2), were isolated from the roots of Notopterygium incisum (Umbelliferae). Their structures were successfully established using spectroscopic techniques, including mass spectrometry (MS), nuclear magnetic resonance (NMR), and electronic circular dichroism (ECD) spectroscopy. In vitro anti-inflammatory assay using a lipopolysaccharide-stimulated RAW264.7 macrophages indicated compounds 1 and 2 showed anti-inflammatory potential by inhibiting the secretion of NO at the concentra-tion of 7.5 μM.
DOI http://doi.org/10.25135/rnp.2511.3718 Keywords Notopterygium incisum phenylpropanoid derivatives nitric oxide anti-inflammatory DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.4) Cytotoxic phloroglucinol glucosides from microalgae (Spirulina platensis)
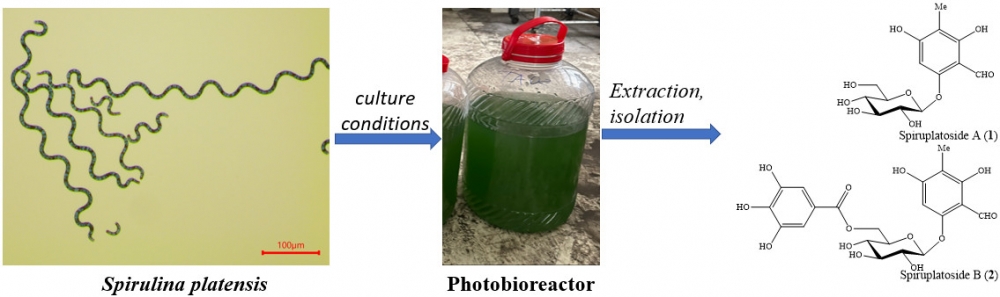
Two new phloroglucinol glucosides, spiruplatosides A (1) and B (2) along with six known compounds, including 5,7-dimethoxyflavone (3), 5-hydroxy-7,4'-dimethoxyflavone (4), 5,7,4'-dimethoxyflavone (5), terephthalic acid dimethyl ester (6), terephthalic acid-(2-hydroxyethyl ester)-methyl ester (7), 13S-hydroxy-9Z,11E-octadecadienoic acid (8) were isolated from the microalgae Spirulina platensis. Compounds 1–8 were evaluated for cytotoxic activity against SK-LU-1 and HepG2 cancer cells. Compounds 1 and 2 were the most active with IC₅₀ values ranging from 1.40 to 3.66 μM. Compounds 4 and 5 showed moderate effects with IC50 values ranging from 66.09 to 74.46 μM.
DOI http://doi.org/10.25135/rnp.2510.3663 Keywords Spirulina platensis microalgae spiruplatoside phloroglucinol glucoside cytotoxic activity DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.5) A new glutarimide compound from Streptomyces sp. JCM 4793
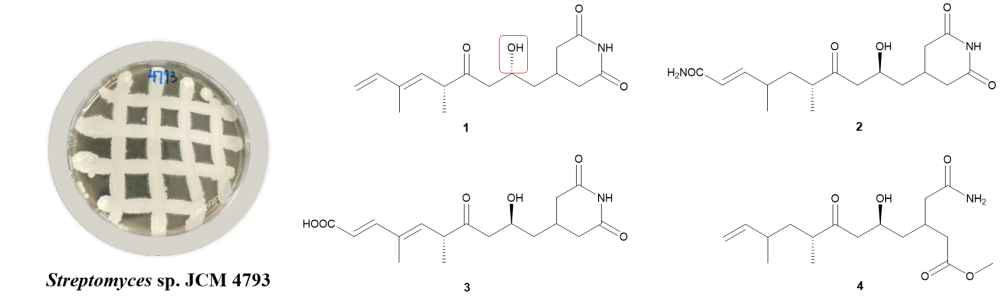
In this study, a previously undescribed glutarylimide compound was isolated from Streptomyces sp. JCM 4793. Its structure was evaluated through examinations of the NMR, HREIMS data and ECD calculation. Compound 1 was measured for its cytotoxicities against A549, K562, HepG2, SW480 and MDA-MB-231 cells. Compound 1 exhibited significant inhibitory activity against both K562 and A549 tumor cell lines, with IC50 values of 4.840 ± 0.329 μmol/L and 6.036 ± 0.157 μmol/L, respectively.
DOI http://doi.org/10.25135/rnp.2510.3694 Keywords Streptomyces glutarimide configuration cytotoxicities DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.6) A new benzoin derivative from the medicinal plant Viscum coloratum
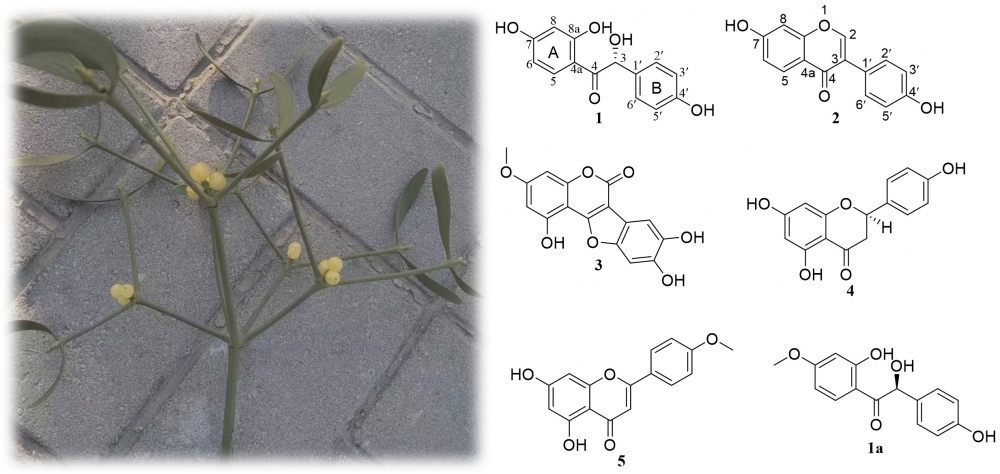
Chemical investigation of Viscum coloratum resulted in the isolation of a new benzoin, named 7-O-demethylamycolabenzoyl (1), along with two known isoflavones (2 and 3) and two known flavones (4 and 5). The structure of 1 was elucidated through extensive NMR and HRESIMS analysis and was further confirmed by 13CNMRcalculations. The absolute configuration at the sole chiral center was assigned as R by comparison of its experimental ECD spectrum with that of the analogue amycolabenzoyl (1a). A plausible biosynthetic pathway for 1 is proposed, suggesting its derivation from the co-isolated isoflavone 2. Compound 1 showed weak α-glucosidase inhibitory effects.
DOI http://doi.org/10.25135/rnp.2203.2375 Keywords Benzoin derivative 7-O-demethylamycolabenzoyl Viscum coloratum DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.7) Exploration of the mechanism of Fuer formula for treating precocious puberty based on transcriptomics and network analysis
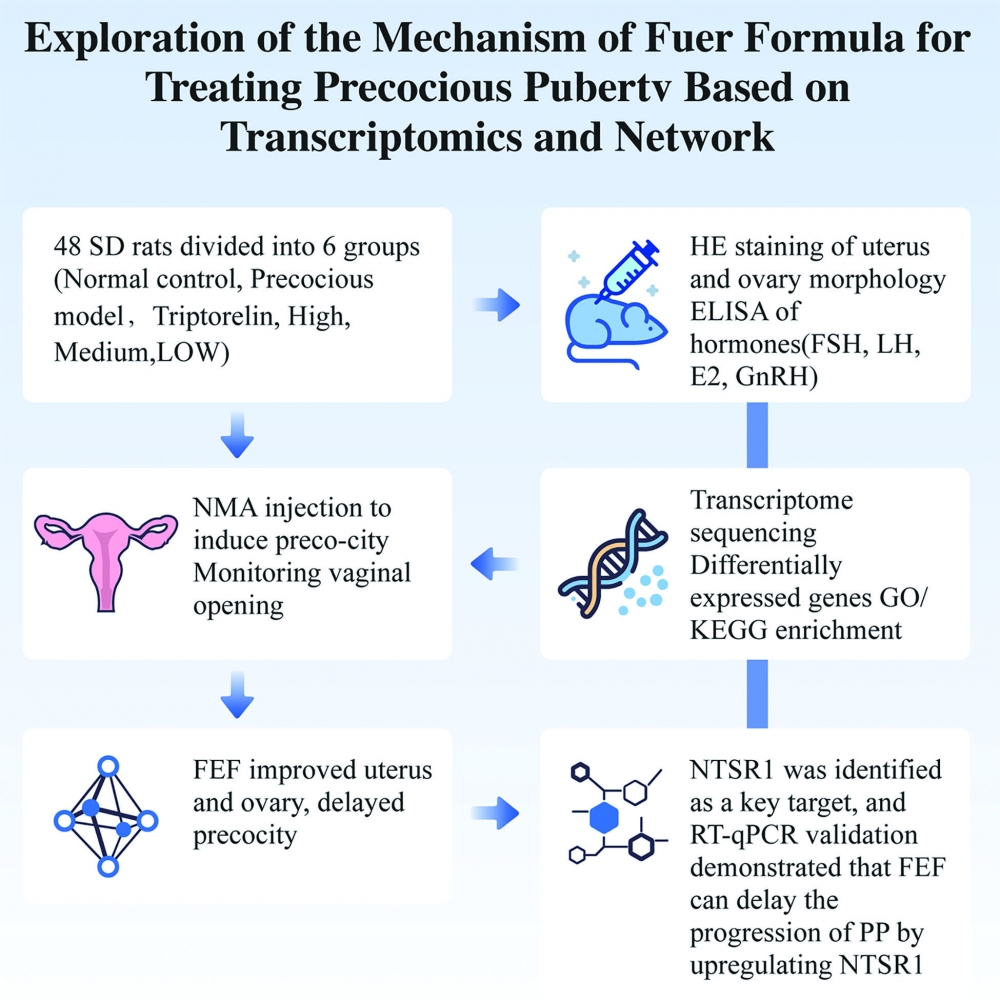
This study investigated the therapeutic mechanism of Fuer Decoction against precocious puberty through transcriptomics and network pharmacology. Female SD rats were allocated to six groups, and an NMA-induced model was established before drug administration. Vaginal opening, estrous cycle, reproductive organ morphology, and serum LH, FSH, E2, and GnRH levels were evaluated. Transcriptome sequencing, differential gene analysis, PPI network construction, GO/KEGG enrichment, and molecular docking were conducted, and NTSR1 was validated via RT-qPCR. UHPLC-MS analysis identified 603 potential active compounds in the decoction. Fuer Decoction alleviated pathological changes and hormonal dysregulation in model rats. NTSR1 emerged as a core target, mainly associated with neuroactive ligand–receptor interactions and calcium signaling pathways.Eicosapentaenoic acid and aurantiamide acetate showed strong binding affinities to NTSR1. These findings suggest that Fuer Decoction may exert therapeutic effects on precocious puberty by modulating these pathways and key genes.
DOI http://doi.org/10.25135/rnp.2510.3665 Keywords Fuer formula molecular docking precocious puberty rats transcriptomics DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.8) Bioactive compounds from the flowers of Cannabis sativa L.: Isolation of a new alkaloid and biological evaluation of cannabinoids and sesquiterpenoids

Cannabis sativa L. is a significant plant widely used for both medicinal and recreational purposes. Previous studies have reported that the secondary metabolites of this plant continue to play a crucial role in drug research and development. This study aims to investigate bioactive compounds in the n-hexane fraction obtained from the ethanolic extract of C. sativa flowers. The isolation yielded eight compounds (1-8), including one new compound (1). Their chemical structures were elucidated by 1D and 2D NMR, HR-ESI-MS, and comparisons with previously reported data. The results of the preliminary biological evaluation revealed that compounds 2-4 and 8 suppressed the proliferation of SK-N-SH cells at 10 μM. Notably, compound 4 displayed the strongest activity, with an IC50 value of 22.53 ± 1.92 μM, suggesting its potential as a candidate for the development of neuroblastoma cell proliferation inhibitors. In addition, compounds 1-8 were also evaluated for antioxidant, tyrosinase, elastase, and collagenase inhibitory activities. Among them, compound 1 showed the highest antioxidant activity, inhibiting 50.89% at 100 μM, compared with ascorbic acid. Compounds 1-3 and 6-8 also demonstrated elastase inhibitory activity, with inhibition rates ranking from 49.95% to 56.68% at 1 mM, relative to oleanic acid as a positive control. Similarly, compounds 1, 3, and 5 inhibited collagenase, with inhibition rates ranging from 55.34% to 73.17% relative to EGCG as a positive control. However, all compounds displayed relatively weak tyrosinase inhibitory effects, with inhibition ranging from 5.22% to 31.02%. This study also represents the first published evaluation of the inhibitory activities of isolated compounds from C. sativa flowers against tyrosinase, elastase, and collagenase.
DOI http://doi.org/10.25135/rnp.2509.3648 Keywords Cannabis sativa cannabinoids SK-N-SH cells tyrosinase elastase collagenase DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.9) Penioxalone A: A new isocoumarine derivative fromPenicillium oxalicum with potent cytotoxic effects on A549 cells
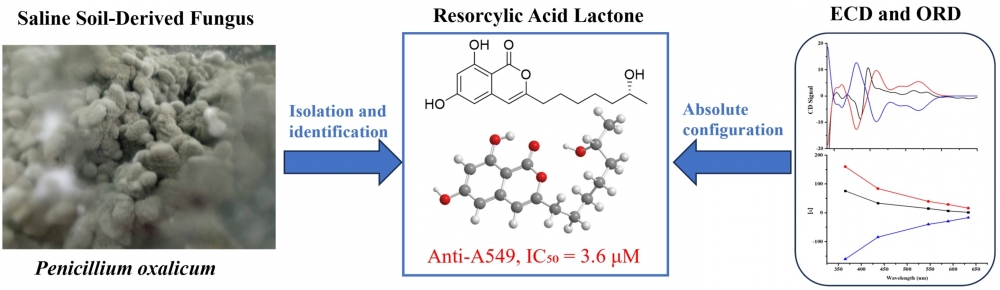
A chemical analysis of Penicillium oxalicum, a fungus isolated from saline soil, resulted in the discovery of four bioactive compounds (1–4), including a new resorcylic acid lactone, named penioxalone A (1). The structural characterization of penioxalone A was achieved through comprehensive spectroscopic techniques, including 1D and 2D NMR spectroscopy, coupled with HRESIMS data. To determine its absolute configuration, experimental data from ECD and ORD were compared with theoretical calculations. The cytotoxicity of compounds 1, 3, and 4 was assessed against A549 human lung carcinoma cells, revealing IC₅₀ values of 3.6, 0.23, and 0.35 μM, respectively. These compounds exhibited enhanced potency compared to cisplatin, a standard chemotherapy drug, which had an IC₅₀ of 4.2 μM.
DOI http://doi.org/10.25135/rnp.2511.3716 Keywords Fungus Penicillium oxalicum penioxalone A resorcylic acid lactone cytotoxic activity DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.10) Two new compounds from the leaves of Forsythia suspensa (Thunb.) Vahl
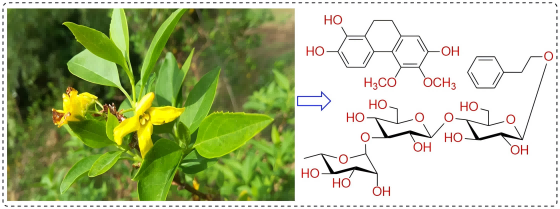
A new phenanthrene derivative and a new phenylethanoid glycoside were isolated from Forsythia suspensa (Thunb.) Vahl leaves, named as 5,6-dimethoxy-9,10-dihydrophenanthrene- 1,2,7-triol (1), phenethyl alcohol 8-O-α-L-rhamnopyranosyl-(1→3)-O-β-D- glucopyranosyl-(1→4)-O-β-D-glucopyranoside (2). These structures were determined by analysing their physical and chemical properties and using high-resolution mass spectrometry, infrared spectroscopy, nuclear magnetic resonance and other spectroscopic techniques. In addition, the toxic activity of the isolated compounds on RAW 264.7 cells was evaluated, but the isolated compounds did not show significant activity.
DOI http://doi.org/10.25135/rnp.2511.3726 Keywords Forsythia suspensa (Thunb.) Vahl phenylethanoid glycoside phenanthrene derivative DETAILS PDF OF ARTICLE © 2026 ACG Publications. All rights reserved.