Organic Communications
Year: 2020 Volume: 13 Issue:4 October-December
1) Cycloaddition reactions of 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione (PTAD) and 4-methyl-3H-1,2,4-triazole-3,5(4H)-dione (MTAD): A short review
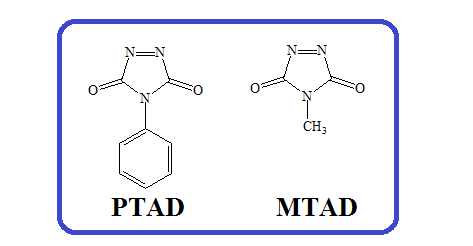
The sources, describing cycloaddition reactions with the participation of 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione (PTAD) and 4-methyl-3H-1,2,4-triazole-3,5(4H)-dione (MTAD) were reviewed from the middle 1970s and they are mentioned in chronological order. It was noted that the most investigated are Diels-Alder reactions and cycloaddition to a few cyclic mono- and dienes. Stereochemistry of obtained products are also described.
DOI http://doi.org/10.25135/acg.oc.88.20.11.1870 Keywords cycloaddition PTAD MTAD Diels-Alder reaction DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.2) Synthesis, spectral characterization, antimicrobial activity and docking studies against DNA Gyrase-A of new 4-chloro-3-nitrobenzene sulfonamide derivatives

A series of new 4-chloro-3-nitrobenzene sulfonamide derivatives 3(a-j) were synthesized from 4-chloro-3-nitrobenzene sulfonyl chloride by reacting various amino acid esters and amines 2(a-j) in high yields. The structures of all the synthesized compounds were characterized by the IR, NMR (1H & 13C), mass and elemental analyses. Further, all the synthesized compounds were tested for the antimicrobial activity and docking studies were carried out with DNA Gyrase-A. Most of the compounds showed good to moderate antimicrobial activities and binding affinity towards DNA Gyrase-A structure.
DOI http://doi.org/10.25135/acg.oc.90.20.11.1858 Keywords 4-Chloro-3-nitrobenzene amino acid esters antimicrobial activity docking studies DNA Gyrase A sulfonamides DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.3) Synthesis of novel potential ROCK inhibitors and their antimigratory effects
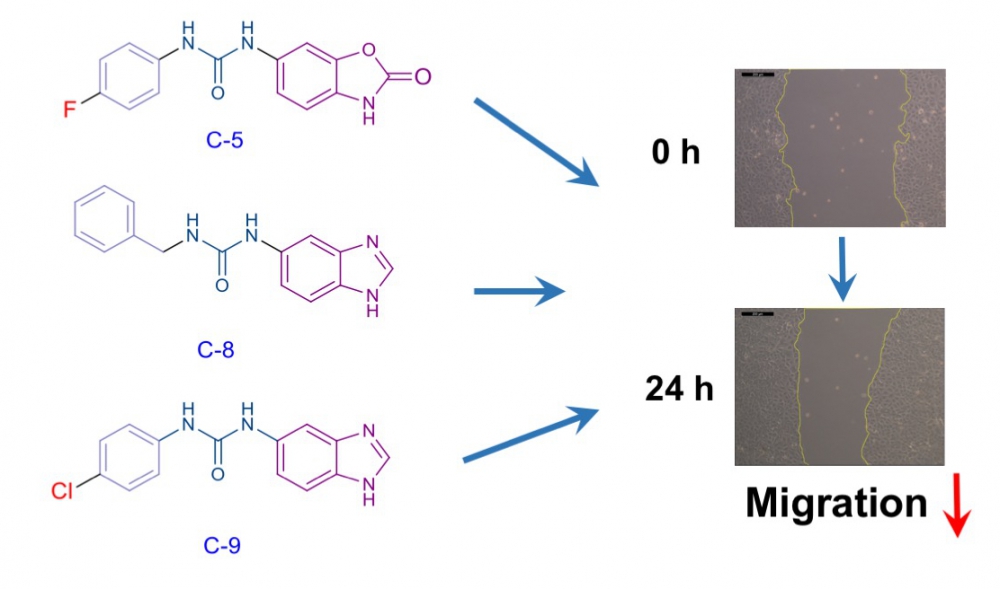
Rho kinase (ROCK), an enzyme belonging to the serine-threonine kinase family, is involved in the regulation of basic cellular processes such as morphology, movement, division, differentiation and apoptosis. On the other hand, excessively activated ROCK can cause to cardiovascular and neurological disorders or cancer. In recent years, overactivation of Rho kinases has been associated with increased metastasis in various tumor types and has been explored as target for the development of new anticancer drugs. We report here the design and synthesis of five urea derivatives in search of novel inhibitors of cancer cell migration. Compounds evaluated for their cytotoxic activities against breast (MCF-7) cancer cell line. After determination of the ineffective concentrations of compounds on the proliferation of MCF-7 cells, wound healing experiments were conducted to investigate the antimigratory effects of compounds. While compounds 4 and 10 had no effect on cell migration, treatment of MCF-7 cells with compounds 5, 8 and 9 resulted in significant reduction in cell motility. Taken together our results suggest that the newly synthesized compounds 5, 8 and 9 had the potential antimigratory activity through possible ROCK inhibition in cancer cells.
DOI http://doi.org/10.25135/acg.oc.87.20.10.1846 Keywords ROCK migration MCF-7 benzoxazolone benzimidazole urea DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.4) Facile microwave synthesis of a novel phenothiazine derivative and its cytotoxic activity
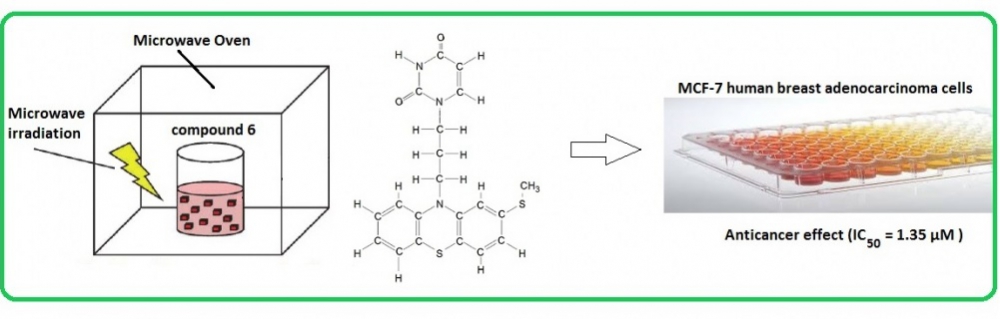
Herein, a facile procedure for microwave synthesis and NMR characterization of phenothiazine derivatives 10-(3-hydroxypropyl)-2-(methylsulphanyl)-10H-phenothiazine (3) (25% yield) and 1-[3-(2-methylsulfanyl-10H-phenothiazine-10-yl)-propyl]pyrimidine-2,4-(1H,3H)-dione (6) (67% yield) is described. After successful microwave synthesis steps, MTT cytotoxicity experiments gave rise to greater anticancer effect of compound 6 in MCF-7 human breast adenocarcinoma cell line (IC50= 1.35 µM) as compared to literature values for tamoxifen (IC50= 8.3 µM) and doxorubicin (IC50= 27 µM).
DOI http://doi.org/10.25135/acg.oc.86.20.10.1853 Keywords Microwave synthesis phenothiazine derivatives 1-(3-(2-methylsulfanyl-10H-phenothiazine-10-yl)-prop-1-yl)pyrimidine-2,4(1H,3H)-dione NMR IC50 MCF-7 DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.5) Synthesis, anticancer activities and molecular modeling studies of some 2-aminonaphtho[2,3-d][1,3]thiazole-4,9-dione derivatives
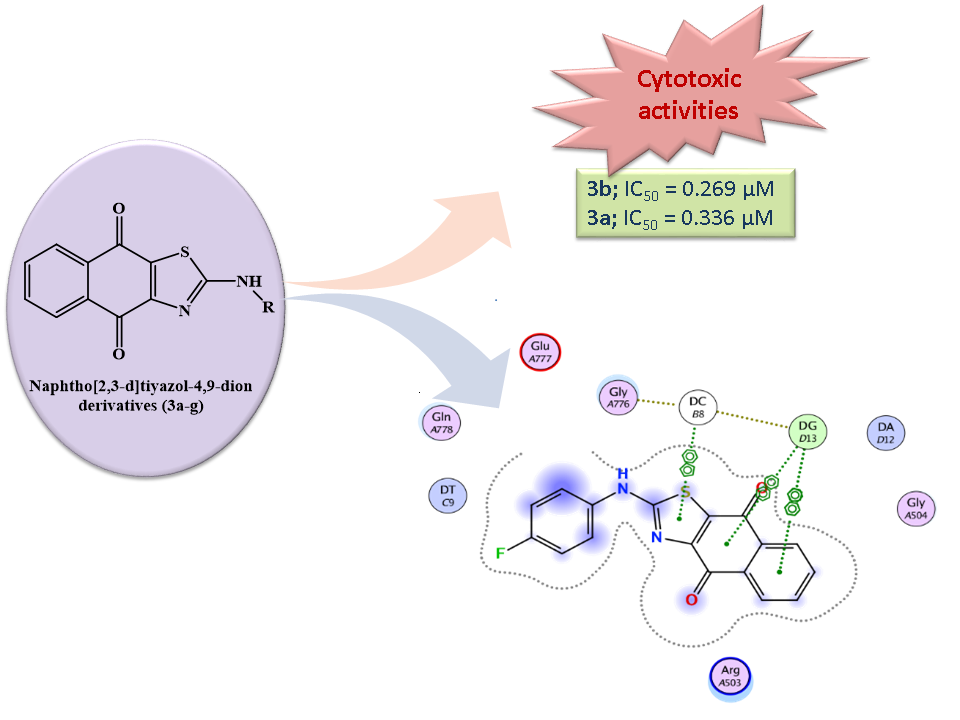
Quinones, especially 1,4-naphthoquinones, are one of the most significant and widely distributed phytochemical groups in nature. 1,4-Naphthoquinones and their synthetic derivatives are found to possess remarkable cytotoxic activities. In this study, a series of 2-aminonaphtho[2,3-d][1,3]thiazole-4,9-dione derivatives were synthesized and their structures were verified with spectral analysis. In vitro cytotoxic activities of the synthesized compounds were evaluated by using MTT assay against MKN-45 (Human Gastric cancer), MDA-MB-231 (Human Breast cancer) and HeLa (Human Cervical cancer) cell lines. Among the synthesized compounds, 3d inhibited MDA-MB-cell proliferation with an IC50 value of 0.276 µM. Compound 3a inhibited HeLa and MKN-45 cell proliferation with IC50 values of 0.336 µM and 8.769 µM, respectively. Compound 3b inhibited HELA cell proliferation with an IC50 value of 0.269 µM. Molecular docking results suggest that the ligands may bind to the hDNA TopoIIβ binding pocket and partially exert their effects. These results propose that 2-aminonaphtho[2,3-d]thiazole-4,9-dion core has important biological effects and further explorations are worthwhile.
DOI http://doi.org/10.25135/acg.oc.91.20.11.1913 Keywords 2-aminonaphtho[2,3-d][1,3]thiazole-4,9-dione naphthoquinones DNA topoisomerases anticancer activity DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.6) Efficient one-pot three-component synthesis of 2H-indazole [2,1-b]phthalazine-1,6,11(13H)-triones at room temperature
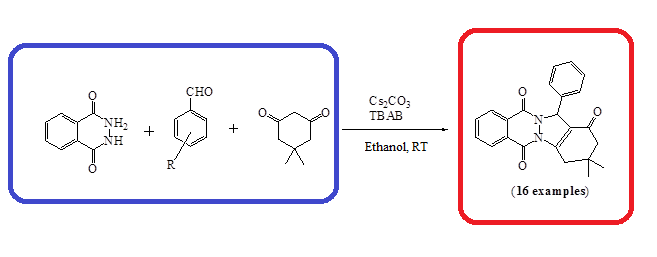
Tetrabutylammonium bromide (TBAB) and cesium carbonate (Cs2O3) catalyzed, one-pot three-component synthesis of 2H-indazole[2,1b]phthalazine-1,6,11(13H)-triones was developed at room temperature in ethanol. Both electron donating and withdrawing groups are compatible under the optimized reaction parameters.
DOI http://doi.org/10.25135/acg.oc.85.20.08.1768 Keywords TBAB; Cs2CO3 indazole[2,1-b]phthalazine-1,6,11(13H)-triones catalysis DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.7) Synthesis of ɣ-glutamyl β-cyanoalanine precursor
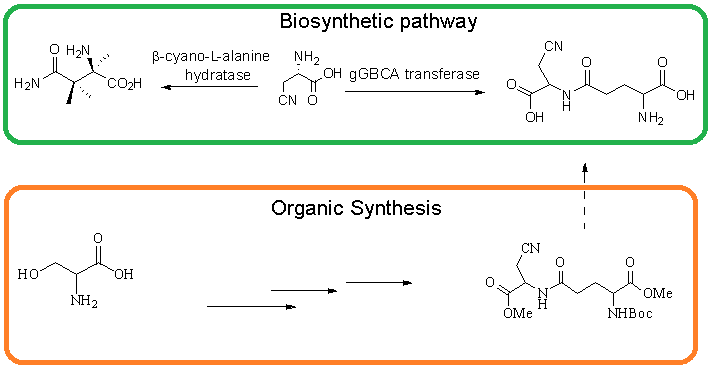
The synthesis of precursors of neurotoxic ɣ-glutamyl β-cyanoalanine was developed starting from L-Serine via through the preparation of b-cyanoalanine and glutamyl units. Coupling of these two intermediates gave methyl ester of ɣ-glutamyl β-cyanoalanine precursor.
DOI http://doi.org/10.25135/acg.oc.89.20.05.1660 Keywords Amino acid β-cyanoalanin (BCA) precursors of gGBCA ɣ-glutamyl β-cyanoalanine (ɡGBCA) DETAILS PDF OF ARTICLE © 2020 ACG Publications. All rights reserved.