Organic Communications
Year: 2022 Volume: 15 Issue:1 January-March
1) Comprehensive review on repurposing of approved medicine in the management of COVID-19 infection
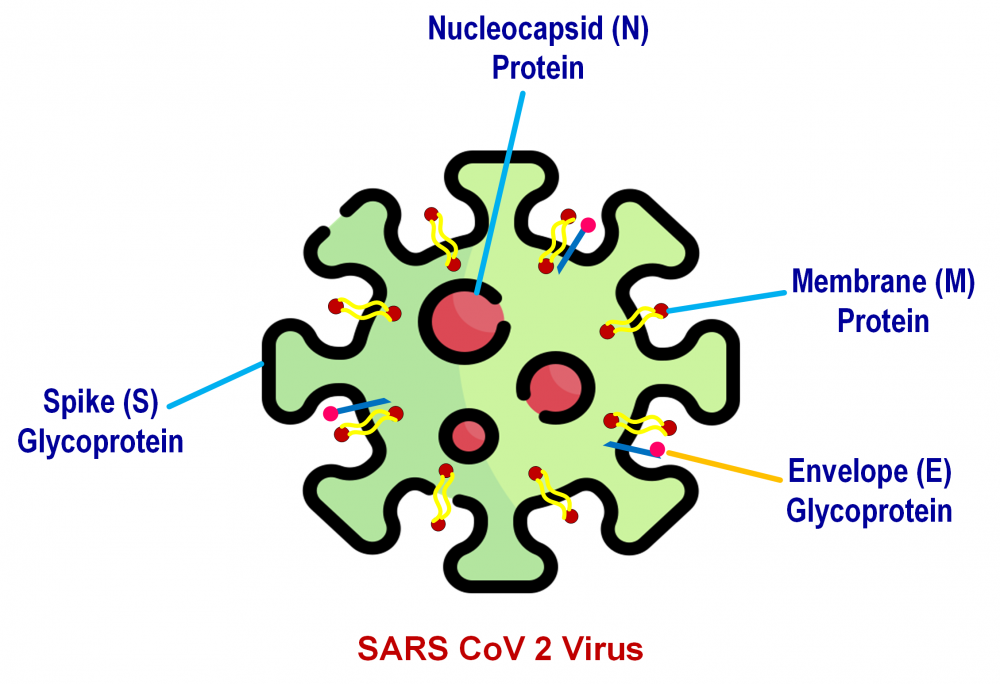
The SARS-CoV-2 virus, accountable for the COVID-19 pandemic, is now sweeping the globe. As a result, as this disease resists testing and adoption of new treatments, repositioning existing medications may provide a quick and appealing method with established safety, features, and dose used. They are not, however, specific or focused. However, numerous medications have been studied for their efficacy and safety in treatment of COVID-19, with the majority currently undergoing clinical trials. The goal is to rapidly expand novel preventative and therapeutic medications, as well as to apply preventive methods such as early patient identification, isolation, and treatment. Moreover, reducing transmission through physical contact is also important. In the fight against this dangerous disease, finding the proper treatment is crucial. This article summarizes several anti-malarial, anti-parasitic, monoclonal antibodies, immunosuppressant, and immunomodulating agents in clinical trials for COVID-19. The purpose of this article is to evaluate and explore the potential roles of several medications now utilized in COVID-19.
DOI http://doi.org/10.25135/acg.oc.117.2110.2244 Keywords COVID-19 SARS-CoV-2 spike protein drug repurposing ACE coronavirus DETAILS PDF OF ARTICLE © 2022 ACG Publications. All rights reserved.2) Acyl glucopyranosides: Synthesis, PASS predication, antifungal activities, and molecular docking
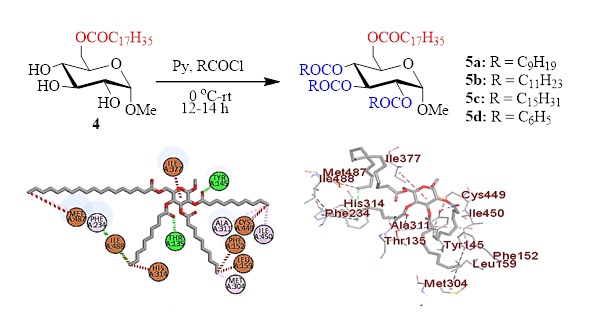
Sugar esters (SEs) with fatty acyl chains showed diverse applications including antimicrobial inhibition against multidrug-resistant (MDR) microorganisms. Thus, fatty acid esters, especially 6-O-stearoyl glucopyranoside ester was prepared by the treatment of glucopyranoside with unimolar stearoyl chloride at low temperature. The 6-O-stearoyl ester thus obtained was further modified to four newer 2,3,4-O-acyl esters to incorporate decanoyl, lauroyl, palmitoyl, and benzoyl chains in the glucopyranoside skeleton. Prediction of activity spectrum for substances (PASS) analyses suggested that these fatty acid esters are more prone to fungal pathogens compared to bacterial pathogens. Guided by PASS analyses in vitro antifungal activities were screened against four fungal pathogens, which supported the PASS observation. To validate the findings molecular docking was conducted with lanosterol 14α-demethylase (CYP51), a significant fungal enzyme, which is the principal target of antifungal drugs. Corroboration of in vitro results with binding affinity revealed the possibility of glucopyranoside-based fatty acyl esters with stearoyl, decanoyl and lauroyl chains as highly potential compared to antifungal azole drugs.
DOI http://doi.org/10.25135/acg.oc.120.2201.2307 Keywords Antimicrobial activities binding affinity docking fatty acid esters D-glucopyranoside gegioselectivity DETAILS PDF OF ARTICLE © 2022 ACG Publications. All rights reserved.3) Mandelic acid: an efficient and green organo-catalyst for synthesis of 2,4,5-trisubstituted Imidazoles under solvent free condition
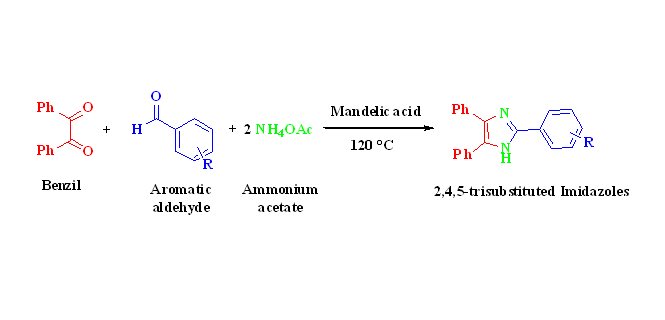
A simple and efficient general procedure has been developed for the synthesis of three-component reaction methodology of 2,4,5-trisubstituted imidazoles by using benzil, ammonium acetate and various aromatic aldehydes. In this methodology, mandelic acid is used as a novel and efficient organo-catalyst in catalytic amount under solvent-free conditions for preparation of variety of 2,4,5-trisubstituted imidazoles derivatives in excellent yields. All products have been characterized by 1H NMR and 13C NMR spectroscopy, IR spectroscopy and mass spectrometry. This method provides versatile advantages of organo-catalyst such as economical, nontoxic, highly stable, readily and easily available as well as solvent-free reaction conditions.
DOI http://doi.org/10.25135/acg.oc.118.22.01.2341 Keywords 2,4,5-trisubstituted imidazoles benzil ammonium acetate mandelic acid organo-catalyst solvent-free DETAILS PDF OF ARTICLE © 2022 ACG Publications. All rights reserved.4) Structural, spectroscopic, Hirshfeld surface and DFT approach of 3,9-dibromophenanthrene
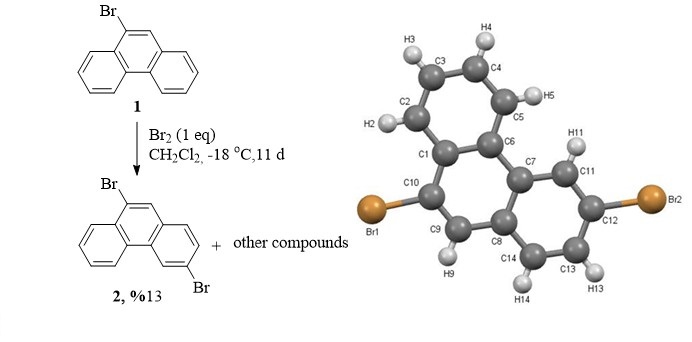
Bromination of 9-bromophenanthrene with one equivalent of bromine resulted in formation of dibromophenantrene isomers. Only 3,9-dibromophenanthrene (2) has been isolated from the mixture and characterized by NMR and X-ray diffraction techniques. Solid state crystal structure of dibromide 2 have been established by X-ray diffraction technique. The Hirshfeld and 2D fingerprint analyses were used to investigate the intermolecular interactions in the crystal structure. The molecular geometries have also been optimized by using density functional theory (DFT-B3LYP) methods with the 6-311G (d,p) basis set and geometric parameters have been compared with the experimental data. Additionally, molecular electrostatic potential (MEP), chemical activity parameters, Fukui function (FF) analysis of compound (2) have been investigated.
DOI http://doi.org/10.25135/acg.oc.119.2109.2213 Keywords Bromophenanthrene DFT chemical activity Hirshfeld surface DETAILS PDF OF ARTICLE © 2022 ACG Publications. All rights reserved.
5) Potassium ferrocyanide promoted an efficient synthesis of benzoxazoles and benzothiazoles under solvent free condition
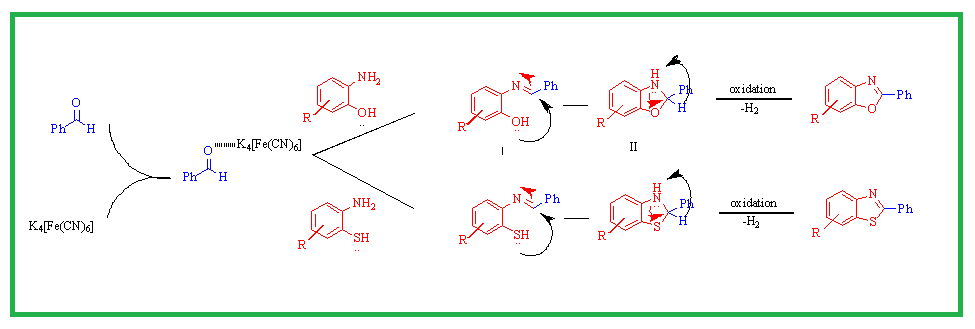
In the family of heterocycles that includes benzoxazoles and benzothiazoles, there exist compounds with a wide range of biological activity. Because of this characteristic, we designed a moderate and effective technique for the synthesis of 2-substituted benzoxazole and benzothiazole using condensation of aldehyde and 2-aminophenol or 2-aminothiophenol via oxidation of carbon-nitrogen bond. Potassium ferrocyanide catalyzed one-pot synthesis is efficient and provides for quick reaction times, simple set-up and high yields. As a result, we provide here a technique for the rapid solvent free synthesis of benzoxazoles and benzothiazoles. Some synthesized products were identified by 1H-NMR, 13C-NMR and MASS. The role of potassium ferrocyanide as a catalyst is represented by plausible reaction mechanism.
DOI http://doi.org/10.25135/acg.oc.121.2110.2242 Keywords Aldehyde potassium ferrocyanide benzoxazoles; benzothiazoles solvent free. DETAILS PDF OF ARTICLE © 2022 ACG Publications. All rights reserved.