Organic Communications
Year: 2025 Volume: 18 Issue:1 January-March
1) Embarking on the 18th year of Organic Communications
2) In memory of Professor George Michael Sheldrick (1942-2025)

This letter of tribute honors the memory of Professor George Michael Sheldrick (1942–2025), a pioneering chemist whose work profoundly shaped the field of structural chemistry. He is best known for developing the SHELX program suite, which revolutionized crystal structure determination worldwide. With over 284,000 citations and an H-index of 113, his scientific legacy is truly remarkable.Professor Sheldrick received numerous prestigious awards, including the Leibniz Prize and a Fellowship of the Royal Society. In 2024, the George M. Sheldrick Prize was established in his name. Remembered as both a brilliant scientist and a compassionate mentor, he leaves behind a legacy marked by excellence and humanity.
DOI http://doi.org/10.25135/acg.oc.184-2503-3471 Keywords Sheldrick X-ray diffraction DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.3) Organic heterocyclic compounds as ionophores: recent progress in potentiometric sensing
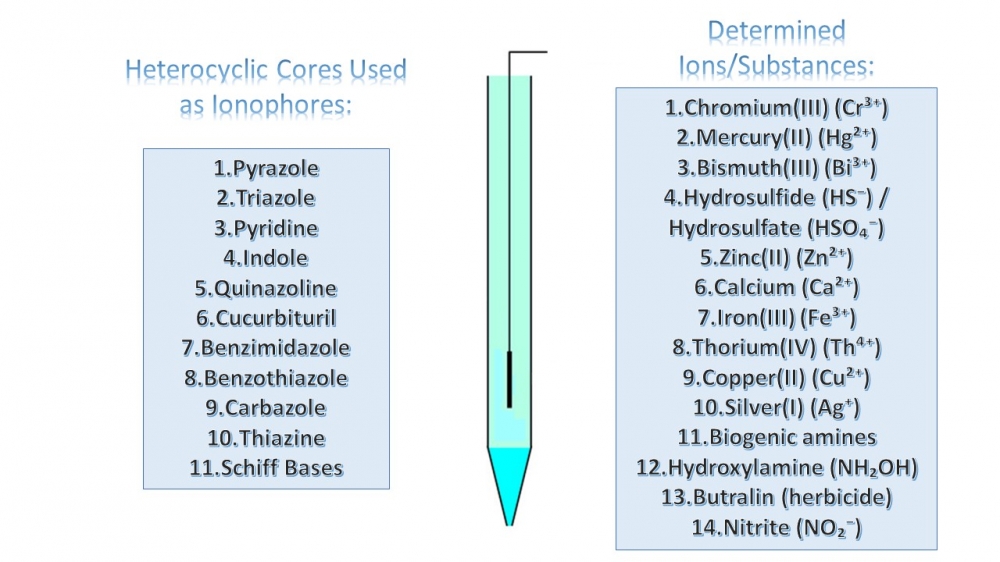
This review article provides a comprehensive analysis of the advancements in organic heterocyclic compounds as ionophores for potentiometric sensors over the past decade. It highlights their critical role in modern analytical chemistry and their impact on sensor performance. Various classes of heterocyclic ionophores—including five-membered monoheterocyclic compounds (e.g., pyrazole and 1,2,4-triazole derivatives), pyridine derivatives, condensed heterocyclic compounds (such as indoles, quinazolines, cucurbiturils, benzimidazoles, benzothiazoles, carbazoles, and thiazines), Schiff bases, and macroheterocyclic compounds—are systematically reviewed. Special emphasis is placed on the design, synthesis, and optimization of these ionophores within polymer-based and PVC membrane electrodes, along with their key performance parameters such as linear concentration ranges, detection limits, response times, and ion selectivity. By analyzing research findings from the last 10 years, this review underscores the advantages of organic heterocyclic ionophores in terms of selectivity, stability, and versatility, making them highly suitable for applications in environmental monitoring, clinical diagnostics, food safety, and industrial analysis. Additionally, emerging trends and ongoing challenges in potentiometric sensor development are discussed, offering insights into future research directions in this rapidly evolving field.
DOI http://doi.org/10.25135/acg.oc.183.2502.3444 Keywords Ionophores potentiometric sensors ion-selective electrodes PVC membrane electrodes sensor selectivity macroheterocyclic compounds DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.4) Baker’s yeast as a biocatalyst for efficient and substrate-selective N-acetylation of anilines
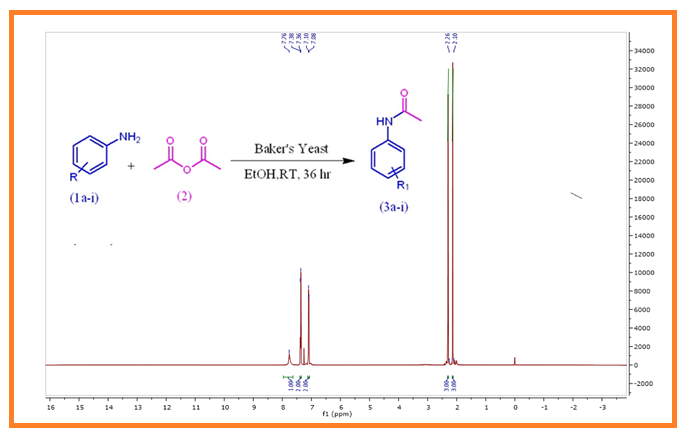
In the present study, we have developed an efficient N-acetylation method of anilines under green and eco-friendly conditions, where Baker’s Yeast (BY) was used as biocatalyst. The results of this study clearly demonstrate towards the N-acetylation of only anilines to form acetanilides (3a-i) with acetic anhydride in good to excellent yield. Interestingly, acetylation of phenol/thiophenol, aliphatic amines/heterocyclic amines and amino acids failed under the same conditions. This N-acetylation reaction with substrate-selective follows simple procedure and requires simple work up. Thus, we can conclude that the present method is a simple, efficient, substrate selective and eco-friendly.
DOI http://doi.org/10.25135/acg.oc.182.2501.3412 Keywords Anilines acetylation acetanilide acedic anhydride Baker yeast bio-catalyst DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.5) Synthesis and molecular docking study of ethyl piperidine-1-carboxylate derivative Schiff bases
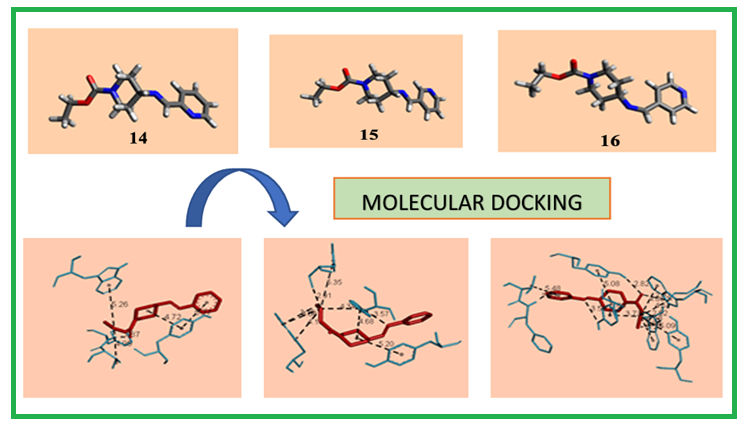
In the present study, new Schiff bases (14-16) were obtained from the condensation reaction of Ethyl 4-aminopiperidine-1-carboxylate and 2/3/4-pyridine carboxyaldehyde compounds. Desired compounds were successfully synthesized with high yields in a short time. Chemical characterization (1H-NMR, 13C-NMR and elemental analysis) and molecular docking studies of the synthesized compounds were performed against the 7XN1 structure (human acetylcholinesterase in complex with tacrine) and reference drug (Donepezil and Tacrine). It was determined that compound 16 (-7.52) had higher binding energy than Tacrine (-7.48), and compounds 14 (-7.34) and 15 (-7.41) showed values close to Tacrine (-7.48). The synthesized compounds can be potent inhibitors for hAChE.
DOI http://doi.org/10.25135/acg.oc.182.2503.3450 Keywords Schiff base microwave molecular docking tacrine donepezil green chemistry DETAILS PDF OF ARTICLE © 2025 ACG Publications. All rights reserved.