Organic Communications
Year: 2023 Volume: 16 Issue:1 January-March
1) Molecular docking and antioxidant activity studies of imidodithiocarbonate derivatives containing pyrimidine
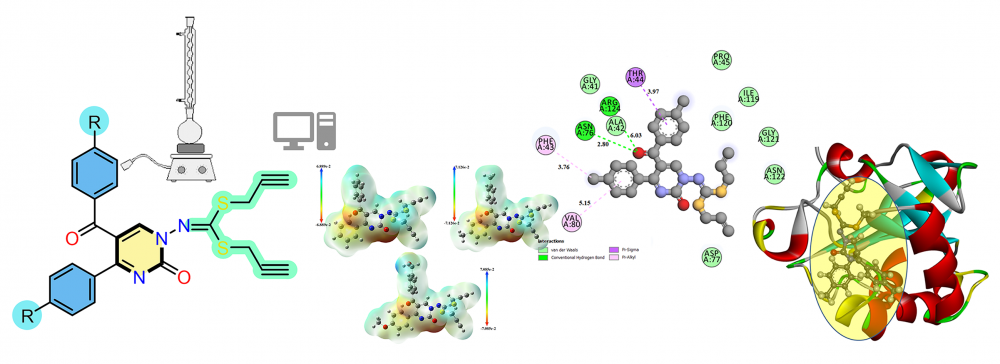
Imidodithiocarbonate derivatives containing pyrimidine ring, potentially functional molecules were synthesized and characterized. FTIR, 1H NMR and 13C NMR spectroscopy and elemental analysis methods were used for characterization. The obtained compounds were screened for their antioxidant capacity using DPPH (1,1-diphenyl-2-picrylhydraziyl) free radical-scaving and ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonicacid) assays. The possible interactions between the molecular docking calculations of the synthesized imidodithiocarbonate derivatives and their binding potentials to the active sites of target enzymes.
DOI http://doi.org/10.25135/acg.oc.143.2212.2658 Keywords Pyrimidine imidodithiocarbonate molecular docking antioxidant activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.2) Synthesis and biological evaluation of new 4-thiazolidinone derivatives of flurbiprofen

In this study, the synthesis and characterization of 2-(2-fluorobiphenyl-4-yl)-N´-[(substituted methylene]propanehydrazides (3a-s) and 2-(2-fluoro-[1,1'-biphenyl]-4-yl)-N-(5-methyl-2-(substituted aryl)-4-oxothiazolidin-3-yl)propanamides (4a-s) are described and also the antiproliferative effect of the compounds on HT-29, HeLa, A549 and MCF-7 cancer cell lines is investigated. Additionally, mouse embryonic fibroblast cells NIH3T3 were also evaluated to determine the selectivity. The results showed that the identified compounds did not cause any toxicity against NIH3T3 cell line. Moreover, N-(2-(3,5-Bis(trifluoromethyl)phenyl)-5-methyl-4-oxothiazolidin-3-yl)-2-(2-fluoro-[1,1'-biphenyl]-4-yl)propanamide (4h) had the most growth inhibitory effect (55.97% inhibition) on HT-29 colorectal adenocarcinoma cell line. The results obtained from the study show that the compound 4h, which has no cytotoxic effect on normal cells, may be an alternative in the treatment of colon cancer.
DOI http://doi.org/10.25135/acg.oc.144.2212.2653 Keywords Hydrazone 4-thiazolidinone MTT antiproliferative activity flurbiprofen DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.3) Synthesis of some piperazine/piperidine amides of chromone-2-carboxylic acid as potential soluble epoxide hydrolase (sEH) inhibitors
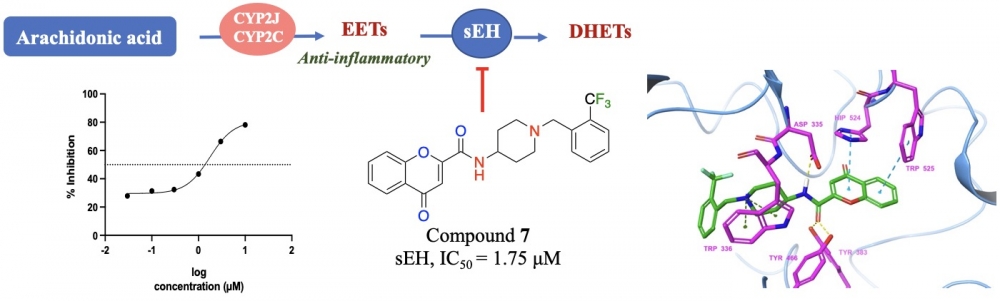
Inhibition of soluble epoxide hydrolase (sEH) is implicated as a new therapeutic approach against inflammatory disorders in the context of metabolic and cardiovascular diseases. In the course of our ongoing research on sEH inhibitors, we synthesized novel piperididine/piperazine amide derivatives of the chromone-2-carboxylic acid, and evaluated their inhibitory properties against human sEH. The chemical structures of the target compounds (2-5, 7-9) were elucidated by means of 1H-NMR, 13C-NMR and HRMS spectra. Initial screening of final compounds against sEH at a final concentration of 10 micromolar led to the identification of compound 7, which inhibited sEH activity in a concentration-dependent manner with an IC50 = 1.75 micromolar. Therefore, this chromen-2-amide derivative 7 decorated with benzyl piperidine on the amide side can be regarded as a novel lead structure, which pave the way of developing new analogues with improved inhibitory activities against sEH.
DOI http://doi.org/10.25135/acg.oc.145.2302.2705 Keywords fluorometric inhibition piperazine piperidine soluble epoxide hydrolase DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.4) Synthesis and biological activities of substituted 1,3,4-oxadiazolines
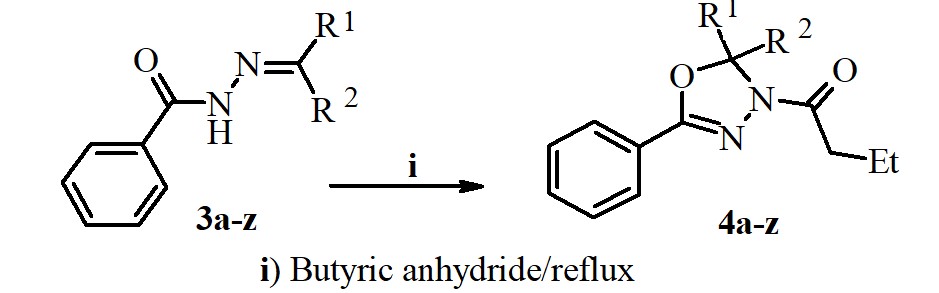
A new series of N-butyryl-1,3,4-oxadiazolines were synthesized via oxidative cyclization reaction of different benzoyl hydrazones with butyric anhydride. The structures of obtained compounds were confirmed by IR, MS, 1H NMR, 13C NMR and Elemental analysis methods and are in full agreement with their molecular structure. The synthesized 1,3,4-oxadiazolines were screened for in vitro for their biological activity against a variety of bacterial strains (Euterococci, Escherichia coli, Staphylococcus aureus, Klebsiella spp, Proteus spp, and fungi (Aspergillus niger, Candida albicans), employing the nutrient agar disc diffusion method. The obtained results showed that these compounds have good inhibition against the tested pathogens.
DOI http://doi.org/10.25135/acg.oc.146.2302.2717 Keywords 1,3,4-Oxadiazolines N-acylhydrazones spiro-oxadiazole oxidative cyclization DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.5) Synthesis and structure analysis of methyl 1-benzyl-5-(phenylamino)methyl)-1H-1,2,3-triazole-4-carboxylate
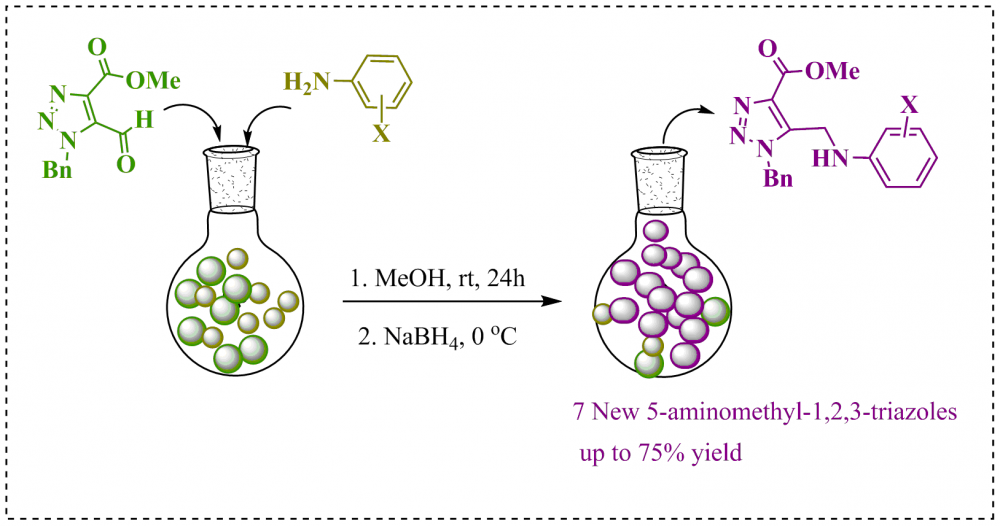
Azole motifs are commonly found in the pharmaceutical industry both as in commercial products and in the literature. In particular, the triazole motifs have many applications such as drug-like and gaining attention with the development of new anticorrosive compounds have made these motifs target molecules. In the literature, it is known that amino-triazole structures have chelating, antibacterial, enzyme inhibition, anti-TB properties, and excellent anticorrosion properties. In this study, the synthesis of novel 1-benzyl-5-(phenylamino)methyl-1,2,3-triazoles (5a-g) that could exhibit both drug-like interaction and corrosion inhibition properties was designed, synthesized and characterized. For this, 5a-g were obtained in one step from reactions of methyl 1-benzyl-5-formyl-1H-1,2,3-triazole-4-carboxylate with anilines carrying different halogen atoms in the 2 and 4 positions. The structures of all the target molecules were fully elucidated by FT-IR, 1H NMR, 13C NMR.
DOI http://doi.org/10.25135/acg.oc.147.2302.2631 Keywords 1,2,3-Triazole 5-formyl-1,2,3-triazole regioselective synthesis 5-(phenylamino)methyl-1,2,3-triazole DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.