Organic Communications
Year: 2024 Volume: 17 Issue:2 April-June
1) A review on some synthetic methods of 4(3H)-quinazolinone and benzotriazepine derivatives and their biological activities
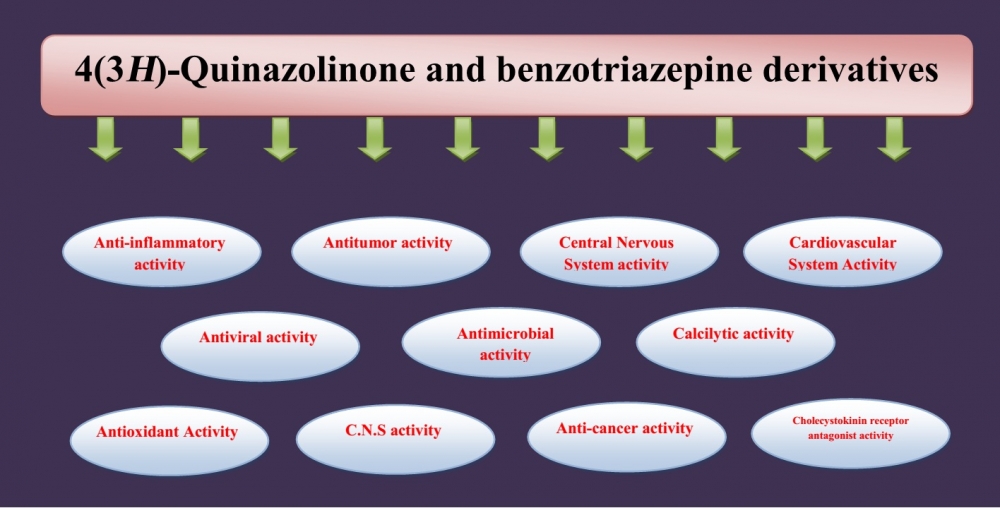
This concise review provides an overview of some synthetic methods utilized for the preparation of 4(3H)-quinazolinone and benzotriazepine derivatives, and explores their diverse biological activities. The review highlights the significance of these compounds in medicinal chemistry and discusses selected synthetic approaches employed in their synthesis. Additionally, it examines the range of biological activities exhibited by these derivatives and briefly discusses their potential applications. This succinct review serves as a valuable resource for researchers interested in the synthesis and biological evaluation of 4(3H)-quinazolinone and benzotriazepine derivatives.
DOI http://doi.org/10.25135/acg.oc.167..2402.3123 Keywords Synthesis heterocyclic compounds 4(3H)-quinazolinone benzotriazepine biological activities medicinal chemistry DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.2) Synthesis of benzhydrol analogues based on 1´-acetoxychavicol acetate (ACA), as a stable and potent antiproliferative agent on breast cancer cell lines, ADMET analysis and molecular docking study.
.png)
Six benzhydrol analogues were successfully synthesised and evaluated for their antiproliferative effect on breast cancer cells. These compounds were designed based on the structure of 1´-acetoxychavicol acetate (ACA) and 1´-acetoxyeugenol acetate (AEA), which known for their anticancer properties. Among them, compounds 3b, 3e, and 3f demonstrated significant activity against MCF-7 and MDA-MB-231 breast cancer cell lines (between 5.5-6.0 µM and 1.1-7.0 µM, respectively), outperforming tamoxifen as the standard control. Molecular docking studies revealed that compounds 3b, 3e, and 3f shows good binding energies ranging from ˗5.13 to ˗7.27 kcal/mol with Nuclear Factor-KappaB Kinase alpha (IκBα) protein (PDB ID: 1NFI), compared to ˗5.27 kcal/mol for tamoxifen (control). 3b and 3f interact with IκBα at several residues including APE77, VAL93, and VAL97. The SwissADME and toxicity prediction analysis indicates that three compounds (3b, 3e, and 3f) adhere to the principles of drug-likeness. These findings suggest that the benzhydrol analogues, particularly 3b, 3e, and 3f, could be promising lead for further development as anticancer agents.
DOI http://doi.org/10.25135/acg.oc.2405.3237 Keywords Benzhydrol analogues antiproliferative breast cancer molecular docking IκBα protein DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.3) Two efficient methods for the total synthesis of the natural diarylheptanoid 7-(4-hydroxyphenyl)-1-phenylheptan-3-one
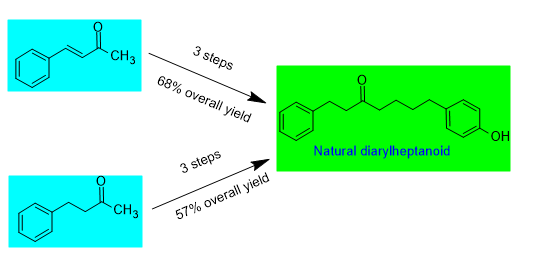
Diarylheptanoids are compounds with an ArC7Ar structure, where two aromatic rings are connected by a seven-carbon chain. To date, over 500 structurally diverse diarylheptanoids have been isolated from nature. These compounds are generally minor secondary metabolites found in plants. Natural diarylheptanoids possess a wide range of biological activities, making their short and efficient total synthesis important for enabling further research on these substances. In this study, two efficient synthesis methods developed for the compound 7-(4-hydroxyphenyl)-1-phenylheptan-3-one, which is isolated as a minor component from Alpinia officinarum, are presented.
DOI http://doi.org/10.25135/acg.oc.169.24.05.3254 Keywords Linear diarylheptanoid 7-(4-hydroxyphenyl)-1-phenylheptan-3-one total synthesis Alpinia officenarum natural product DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.4) Efficient synthesis of benzylidene semicarbazones from aromatic aldehydes by urea-hydrogen peroxide (UHP)
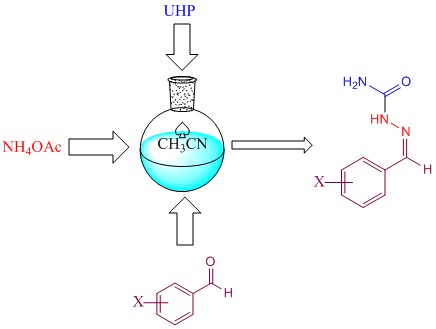
A facile and useful method for synthesis of 1-benzylidene semicarbazones was introduced in this work. The reaction of synthesis of the corresponding 1-benzylidene semicarbazone derivatives is carried out in the presence of acetonitrile-UHP/NH4OAc. In this method, NH4OAc was used as a source of ammonia and urea-hydrogen peroxide (UHP) as reagent. The key advantages of this process are elimination of semicarbazide, good yields and cheap reagents. All products were characterized by common techniques (infrared, 1H NMR, and melting point).
DOI http://doi.org/10.25135/acg.oc.166.2403.3177 Keywords benzylidene semicarbazone UHP aromatic aldehydes acetonitrile DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.5) Eco-friendly synthesis of new thiophene-based Schiff bases containing piperidine rings
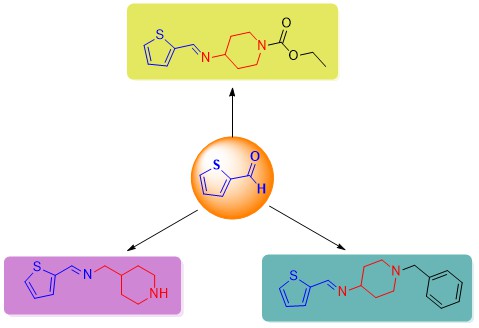
It is known that compounds containing sulfur in their structure have a wide range of biological activities, such as antibacterial, antiallergic, antimicrobial, anticancer, and anticonvulsant. In addition, nitrogen-containing heterocyclic compounds are also found in nature and the structure of drugs. Various compounds, such as piperidine and its derivatives, are also widely used in the synthesis of many drugs. Various Schiff bases have been synthesized in drug development studies, and they have been used in clinical applications as drugs and drug candidates. In this context, the synthesis of new thiophene-based Schiff bases containing piperidine rings is aimed. Green chemistry objectives were adhered to in the syntheses. Thiophene-2-carbaldehyde and piperidine derivatives were used as starting compounds, and new compounds containing thiophene-based piperidine were synthesized without adding any catalyst or solvent to the reaction medium. The desired Schiff base compounds were successfully synthesized in high yield and in a short time.
DOI http://doi.org/10.25135/acg.oc.170.24.06.3246 Keywords Schiff base thiophene piperidine biological activitiy microwave green chemistry DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.