Records of Natural Products
Year: 2024 Volume: 18 Issue:6 November-December
1) Phytochemistry, Pharmacology and Clinical Applications of Cortex Daphnes:A Review

Cortex Daphnes, a traditional Chinese medicine, has been utilized in China for millennia owing to it’s anti-inflammatory and analgesic properties. As it is belonging to the Thymelaeaceae family within the genus Daphne, it has traditionally been employed to dispel wind, eliminate dampness, alleviate pain, and dissipate blood stasis. Consequently, numerous scholars both domestically and internationally have investigated it’s chemical composition, pharmacological effects, and various other aspectsAmong these, daphnetin stands out as the primary active constituent in C. Daphnes, holding significant value as a precursor molecule for drug development. Pharmacological research has demonstrated that compounds and extracts derived from C. Daphnes exhibit notable activities, including anti-inflammatory, anti-tumor, antibacterial, immunomodulatory, antioxidant, and analgesic effects. Clinically, C. Daphnes has a long-standing history of use in the treatment of rheumatic diseases. This presents considerable potential for further development and exploration. According to existing records, C. Daphnes exhibits low toxicity; therefore, refining its processing technology, conducting toxicological studies, and establishing a comprehensive quality standard system are current challenges that need to be addressed. In this review, the botany, traditional uses, phytochemistry, pharmacology, and clinical applications of C. Daphnes, with the aim of providing a valuable reference for its future development and resource utilization was summarized.
DOI http://doi.org/10.25135/rnp.475.2405.3230 Keywords Cortex Daphnes botany phytochemistry pharmacology clinical applications adverse effects and toxicology DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.2) Differentiation of Achillea millefolium, A. crithmifolia, and A. nobilis through Analysis of Volatile Constituents using HS-SPME-GC/MS and Chemometric Techniques
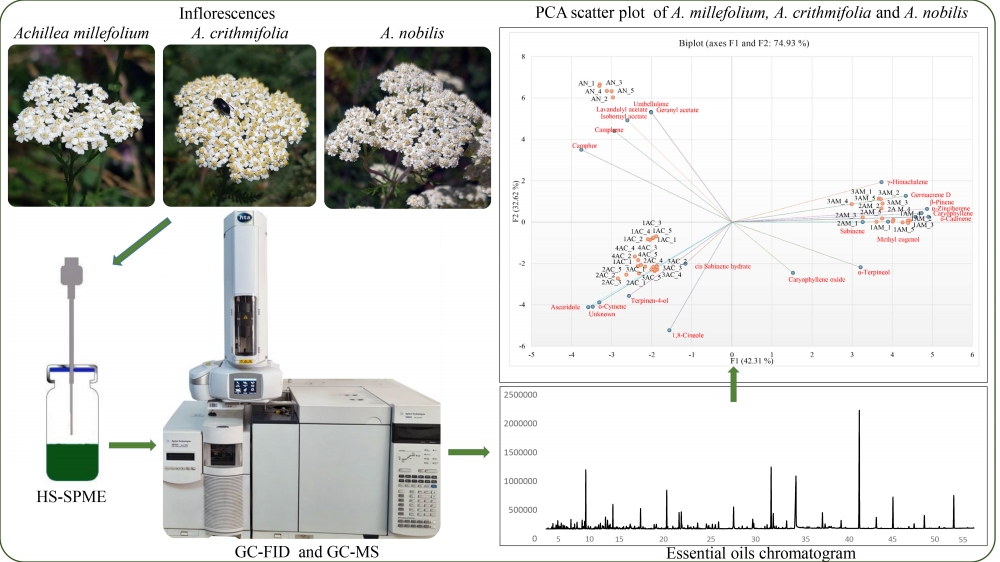
Achillea millefolium, A. crithmifolia and A. nobilis are distinct species that share morphological similarities, making their differentiation challenging. This study utilized gas chromatography-mass spectrometry (GC/MS) combined with headspace-solid phase microextraction (HS-SPME) to analyze the volatile components of these species and employed chemometric methods for species differentiation. A total of 109 volatile compounds from the three studied species were identified. Of these compounds, 71 were identified in A. millefolium, 75 in A. crithmifolia, and 58 in A. nobilis. The primary volatile compounds of A. millefolium were germacrene D, 1,8-cineole, sabinene, and β-pinene; in A. crithmifolia, the main compounds were caryophyllene, 1,8-cineole, camphor, ascaridole, and ο-cymene, while in the essential oil of A. nobilis, camphor, lavandulyl acetate, camphene, and isobornyl acetate were determined as the main volatile compounds. The study demonstrated that the HS-SPME-GC/MS techniques, combined with chemometric methods such as discriminant analysis (DA) and principal component analysis (PCA), effectively distinguished the samples of A. millefolium, A. crithmifolia, and A. nobilis based on the differences in the chemical composition of their essential oils.
DOI http://doi.org/10.25135/rnp.482.2408.3286 Keywords Achillea millefolium A. crithmifolia A. nobilis HS-SPME-GC/MS discriminant analysis (DA) principal component analysis (PCA) DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.3) GLUT4 Translocation Active Flavonoids from Caragana jubata
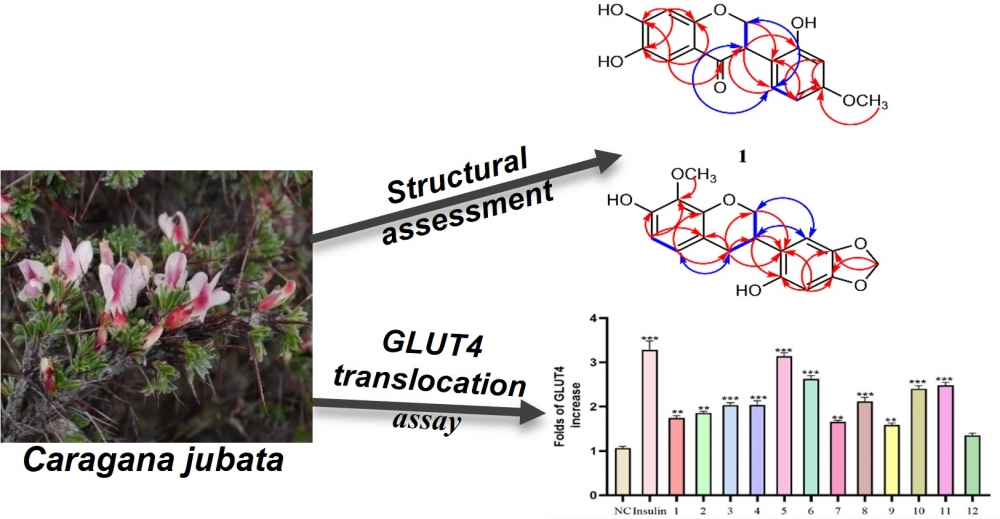
Two new isoflavones, caraganin E (1) and caraganin F (2), were purified from 75% ethanolic extract of Caragana jubata roots, in company with ten known compounds. The chemical structures of two novel isoflavones were elucidated by NMR, HR-ESIMS and circular dichroism (CD). GLUT4 translocation activity was tested in L6 cells for all isolated compounds. Among them, compound 5 exhibited the best activity, increasing the fluorescence intensity by 3.25 folds. The results of the exploration may help us understand the chemotaxonomic variety of natural products in Caragana jubata and enhance the diversity of flavonoids.
DOI http://doi.org/10.25135/rnp.484.2408.3301 Keywords Caragana Caragana jubata flavonoids GLUT4 translocation DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.4) Chemical Composition and Antimicrobial Activity of Essential Oils Obtained from Inula germanica L.
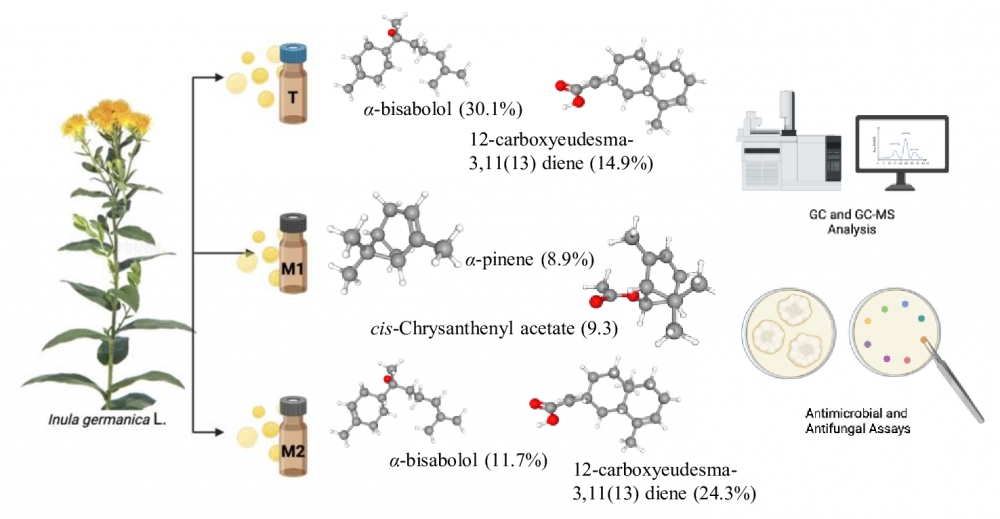
The genus Inula (Asteraceae), consisting of over 100 species predominantly found in Asia, Europe, and Africa, is recognized for its antimicrobial properties. This study explores the chemical composition and antimicrobial activity of essential oils obtained from the aerial parts of Inula germanica L. through conventional (T) and modified (M1, M2) hydrodistillation methods. Using gas chromatography and gas chromatography-mass spectrometry, the conventional method (T) yielded α-bisabolol (30.1%) and 12-carboxyeudesma-3,11(13)-diene (14.9%) as major compounds. The modified method fraction M1 enriched monoterpenes such as trans-verbenol (9.5%), 1,8-cineole (9.5%), and cis-chrysanthenyl acetate (9.3%), whereas M2 was characterized by higher levels of sesquiterpenes like 12-carboxyeudesma-3,11(13)-diene (24.3%) and α-bisabolol (11.7%). The essential oils exhibited moderate to weak antimicrobial activity against tested bacterial and Candida strains, with minimum inhibitory concentrations (MICs) ranging from 125 to 2000 µg/mL. Among all studied samples, M1 showed the best antimicrobial activity against Candida strains in the MIC range of 125-500 µg/mL.
DOI http://doi.org/10.25135/rnp.24.11.3368 Keywords Inula essential oils hydrodistillation methods DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.5) Essential Oil Composition and Antibacterial Activity of Trixis michuacana Lex. var. michuacana and Trixis michuacana var. longifolia

The Trixis genus (Asteraceae) is involved in traditional medicine from Latinoamerica. In Mexico, Trixis michuacana Lex. var. michoacana and T. michuacana var. longifolia are used in the P'urhépecha ethnomedicine. The analysis of their volatile compounds by gas chromatography/mass spectrometry (GC-MS) is described herein. The sesquiterpene compounds were the main constituents. β-Caryophyllene (10.80%), germacrene D (7.93%), β-elemene (7.42%), and γ-elemene (7.37%) highlighted from var. michuacana, while β-elemene (10.02%), α-copaene (9.91%), β-caryophyllene (8.97%), germacrene D (7.40%), and γ-elemene (6.89%) were the major constituents from var. longifolia. A systematic analysis of the chemical components provided an approach to the biosynthetic pathway for volatile metabolite production. Antimicrobial assays provided scientific support for the traditional use of the plants. In-vitro assays using Escherichia coli (ATCC 25922), E. coli (82MR), Klebsiella pneumoniae (ATCC 13883), Pseudomonas aeruginosa (ATCC 27853), Staphylococcus aureus (ATCC 29213), S. aureus (23MR), S. epidermidis (ATCC 12228), S. epidermidis, Candida albicans (17MR), C. glabrata, and C. tropicalis were achieved. These assays revealed MIC values >2 mg/mL when observed bacterial inhibition for both Trixis species, except for var. michuacana against S. epidermidis ATCC 12228, which was 0.5 mg/mL. These results could partially justify the use of the studied plants in ethnomedicine.
DOI http://doi.org/10.25135/rnp.485.2409.3319 Keywords Trixis michuacana Asteraceae antimicrobial activity biosynthetic approach DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.6) Evaluation of the Antioxidant, Antidiabetic and Anti-Alzheimer Effects of Capsella bursa-pastoris-Polyphenolic Profiling by LC-MS/MS
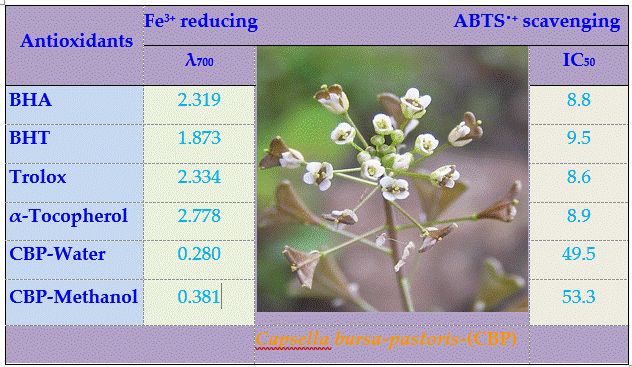
Capsella bursa-pastoris species of the Capsella herb were examined in this study for sreening of antioxidant, antidiabetic and anti-Alzheimer effects. Traditionally, people consumed C. bursa-pastoris against famine and satisfy their hunger. C. bursa-pastoris species have been shown to have utility as food, medicine, and industrial materials after extensive investigation. The antioxidant properties of methanol and water extracts of C. bursa-pastoris species were assessed using; Fe3+-2,4,6-tris(2-pyridyl)-S-triazine (TPTZ), ferric (Fe3+), and cupric (Cu2+) iobs reducing assays, as well as 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid radical (ABTS•+) and N,N-dimethyl-p-phenylenediamine (DMPD) radical scavenging activities. The antioxidant and reducing capacity of C. bursa-pastoris species aerial parts water and methanol extracts were compared with standard antioxidants. The phenolic and flavonoid contents in methanol and water extracts of C. bursa-pastoris species were measured 6.86 to 12.00 mg GAE/g and 61.67 to 145.0 mg QE/g, respectively. The inhibitions of effects of water and methanol extract of C. bursa-pastoris species against α-amylase, and acetylcholinesterase (AChE) enzymes were investigated. The IC50 values were found as 168.6 to 238.6 μg/mL against α-amylase and 19.0 to 20.9 μg /mL against AChE. The number of fenolik compounds in both extracts of C. bursa-pastoris species were recorded using LC-MS/MS. The major phenolic and flavonoid components detected in methanol extract of C. bursa-pastoris were quinic acid (22.629 mg/g), chlorogenic acid (3.211 mg/g), rutin (1.930 mg/g), hesperidin (0.893 mg/g), and isoquercitrin (0.783 mg/g). In a similar order, quinic acid (11.356 mg/g), cyranoside (9.463 mg/g), chlorogenic acid (6.072 mg/g), hesperidin (5.912 mg/g), and isoquercitrin (5.364 mg/g) were found as plentyfull pehenolic antioxidants in methanolic extract. The results clearly demonstrated that polyphenolic antioxidants-rich ingredients of the aerial parts of C. bursa-pastoris species are biological phenolic comounds have persuasive usage in the medication of diabetes and Alzheimer's diseases.
DOI http://doi.org/10.25135/rnp.489.2410.3353 Keywords Capsella bursa-pastoris nzyme inhibition antioxidant activity α-amylase acetylcholinesterase phenolic compounds DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.7) Anti-inflammatory Constituents Isolated From Launaea sarmentosa Against Infection by LPS-stimulated Macrophages

Inflammation is one of the basic pathological processes due to the adaption of immune system against infection or irritation. Since non-steroidal anti-inflammatory drugs (NSAIDs) cause many side effects, investigation of anti-inflammatory natural products promising to provide novel therapeutic agents. Here, Launaea sarmentosa (Willd.) Kuntze, a medicinal herb applied to treat inflammation diseases, was investigated for its anti-inflammatory components to find new therapies for inflammatory syndromes. This study indicated that ethyl acetate fractional extract reduced the expression of pro-inflammatory cytokines, representing the highest activity for both NO radical-scavenging and NO secretion inhibition during LPS-stimulated RAW264.7 macrophages. Besides, five anti-inflammatory compounds, including succinic acid, quercetin, 2(4-hydroxyphenyl)acetic acid, luteolin-7-O-β-D-glucopyranoside, and quercetin-3-O-rutinoside, were isolated and elucidated the structure according to 1D and 2D- NMR. Among these compounds, succinic acid and 2(4-hydroxyphenyl)acetic acid were first reported in this species. Moreover, this study indicated that the presence of these compounds, typically quercetin and luteolin-7-O-β-D-glucopyranoside, enhanced anti-inflammatory ability via deactivation of NF-кB/MAPK pathway to mitigate the expression of IL-6. Hence, this study contributed the initial evidence of anti-inflammatory constituents from Launaea sarmentose and highlighted an approach to discovering natural items or phytotherapeutic agents.
DOI http://doi.org/10.25135/rnp.487.2410.3343 Keywords Anti-inflammatory Launaea sarmentosa mitogen-activated protein kinase nuclear factor kappa B DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.8) Quantification of Secondary Metabolites of Satureja pilosa (Lamiaceae) by LC-HRMS and Evaluation of Antioxidant and Cholinergic Activities
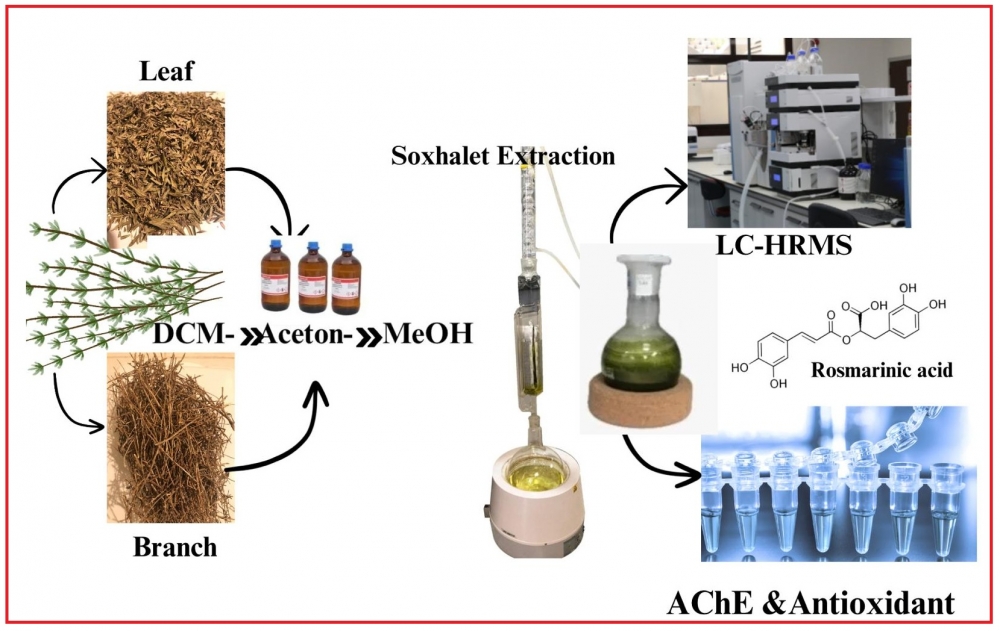
In this study, secondary metabolite profiles of dichloromethane, acetone and methanol extracts of leaves and branches of Satureja pilosa species were determined by LC-HRMS. In-vitro inhibition activities of the obtained extracts against Alzheimer's disease (AD) which is a neurodegenerative disease were performed, and structure-activity relationships were discussed. While rosmarinic acid was found to be at the highest rate among the reported secondary metabolites in acetone and methanol extracts of the plant, penduletin was the secondary metabolite with the highest concentration in dichloromethane extract of the plant. The highest AChE inhibition was determined in leaf methanol extract with IC50: 41.2 ± 5.6 μg/mL, and the highest BChE inhibition was detected in leaf dichloromethane extract of the species with IC50: 52.3 ± 8.6 μg/mL concentration. Herein, total antioxidant capacities of the extracts were determined and reported by DPPH and ABTS radical scavenging and CUPRAC reducing ability methods.
DOI http://doi.org/10.25135/rnp.489.2410.3378 Keywords Satureja pilosa secondary metabolite LC-HRMS AChE BChE antioxidant DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.9) A New Highly Oxygenated Isocoumarin from the Fungus Penicillium sp.
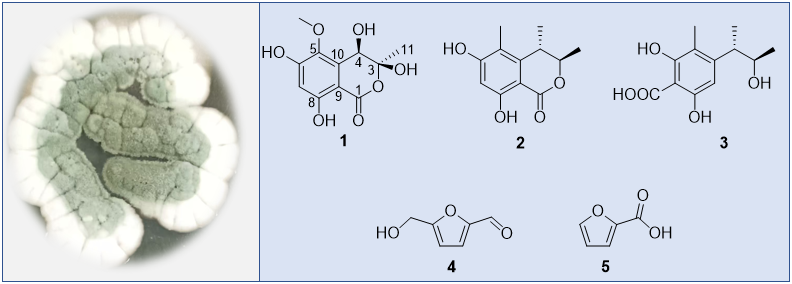
Penicillium is a significant source of bioactive compounds, with the well-known antibiotics penicillin and griseofulvin derived from P. chrysogenum and P. griseofulvum, respectively. In our study, the fungus Penicillium sp. was isolated from shallow-sea sediments. The ethyl acetate extract of this strain exhibited moderate inhibitory effects against Escherichia coli. Subsequent isolation and purification of the extract led to the identification of five compounds (1-5), including a new highly oxygenated isocoumarin and two analogues (2 and 3), as well as two furan derivatives (4 and 5). The structure determination of compound 1 was conducted by extensive analysis of spectroscopic data, including 1H and 13C NMR, as well as 2D NMR techniques (HSQC, COSY, HMBC, and NOESY), in addition to HRESIMS. The known compounds 2-4 were identified as decarboxydihydrocitrinone (2), phenol A acid (3), 5-(hydroxymethyl)furfural (4), 5-hydroxymethyl-2-furancarboxaldehyde (5) by comparing their 1H and 13C NMR data with those reported in the literature. compound 1 exhibited an MIC value of 64 mg/mL toward Escherichia coli.
DOI http://doi.org/10.25135/rnp.481.24.08.3314 Keywords Penicillium sp. isocoumarin fungus isolation structure elucidation DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.10) A New Benzofuran from the Roots of Eupatorium chinense L. and Its α-glucosidase and PTP1B Inhibitory Activities
_graphic_abstract.png)
A new benzofuran, named eupbenzofuran A (1), along with four known compounds, 10,11-dihydroxy-10,11-dihydroeuparin (2), 5-[1'-hydroxyethyl]-2-1'-hydroxyisoprolyl]-benzofuran (3), 3α,6-dihydroxytremetone (4), odoratin (5) were isolated from the roots of Eupatorium chinense L. The structure of compounds were identified by NMR, MS, CD and other spectroscopic methods and comparisons with relevant literature data. Compound 1 had favorable dual inhibitory activities against α-glucosidase and protein tyrosine phosphatase 1B (PTP1B). However, compounds 2-5 had no significant inhibitory activities effects on α-glucosidase and PTP1B (IC50>50 μg/mL). Molecular docking technique was used to calculate the interaction of compound 1 with α-glucosidase and PTP1B, respectively, and the calculated results showed that compound 1 had both strong binding to α-glucosidase and PTP1B.
DOI http://doi.org/10.25135/rnp.483.2408.3280 Keywords Eupatorium chinense L. chemical constituents antidiabetic activity α-glucosidase protein tyrosine phosphatase 1B DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.11) Peniciloxatone A, a New Polyoxygenated Ergostane Steroid Isolated from the Marine Alga-Sourced Fungus Penicillium oxalicum 2021CDF-3
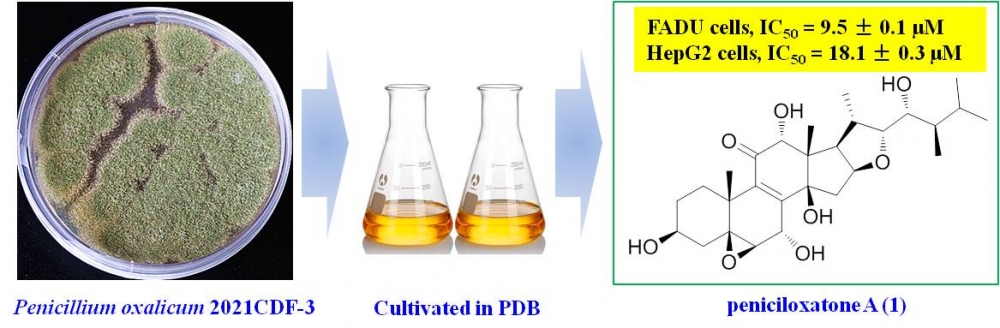
Chemical investigations on the culture of Penicillium oxalicum 2021CDF-3, a marine red alga-sourced endophytic fungus, revealed a new steroid, namely peniciloxatone A (1). With the aid of high-resolution electrospray ionization mass spectrometry (HRESIMS) and nuclear magnetic resonance (NMR) analyses, the structure of peniciloxatone A (1) was determined to be a polyoxygenated ergostane steroid. Cytotoxic activities of 1 were tested against A549, FADU, and HepG2 cells, and 1 was active against the FADU and HepG2 cells, with IC50 values of 9.5 ± 0.1 and 18.1 ± 0.3 μM, respectively.
DOI http://doi.org/10.25135/rnp.486.2410.3339 Keywords Penicillium oxalicum secondary metabolites natural product steroid cytotoxicity DETAILS PDF OF ARTICLE © 2024 ACG Publications. All rights reserved.