Organic Communications
Year: 2023 Volume: 16 Issue:3 July-September
1) Synthesis and biological evaluation of [1,2,3]triazolo[4',5':3,4]pyrrolo[1,2-a] indoles: one-pot reaction under microwave irradiation
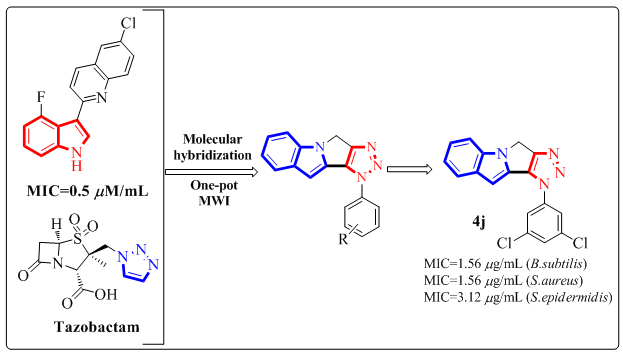
The synthesis and structural determination of several new fused [1,2,3]triazolo-[4',5':3,4]pyrrolo-[1,2-a]indole derivatives (4a-4p) utilising 1HNMR, 13CNMR, and mass spectrum analysis were discussed. The in vitro antibacterial activity of the compounds (4a-4p) against three gramme positive bacterial strains such as B.subtilis, S.aureus, and S.epidermidis revealed that the compounds 4h, 4j, and 4k demonstrated greater activity than the remaining compounds. Compounds 4d, 4i, and 4l had comparable activity to the standard. The antioxidant activity screening findings show that compounds 4c, 4d, and 4j have higher activity than conventional Trolox. Compounds 4b, 4h, 4k, and 4l have high activity, whereas the remaining compounds have moderate to low activity.
DOI http://doi.org/10.25135/acg.oc.155.2307.2833 Keywords Indole fused 1,2,3-triazoles MWI antibacterial activity antioxidant activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.2) Asymmetric transfer hydrogenation of a new N-tosyltetrahydrocarbazole-1-one ester with the Noyori-Ikariya catalyst
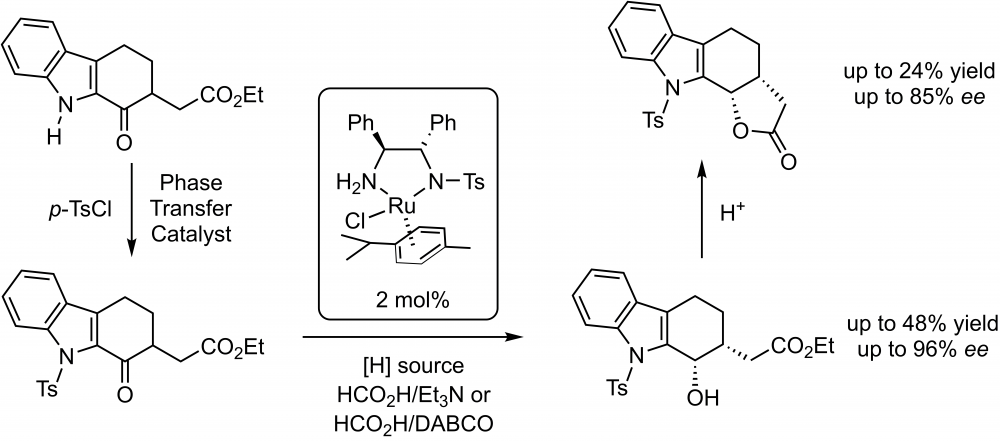
Enantioselective reduction of a new 1-oxotetrahydrocarbazole compound (ethyl 2-(1-oxo-9-tosyl-2,3,4,9-tetrahydro-1H-carbazol-2-yl)acetate) through asymmetric transfer hydrogenation by using the commercially available Noyori–Ikariya ruthenium catalyst and HCO2H/Et3N or HCO2H/DABCO as the hydrogen source have been investigated. High enantiomeric excesses (up to 96% ee) and moderate to good yields (24–72%) for corresponding alcohol and lactone compounds have been achieved. Structures of all compounds have been characterized by spectroscopic techniques.
DOI http://doi.org/10.25135/acg.oc2307.2838 Keywords Asymmetric transfer hydrogenation Noyori–Ikariya ruthenium catalyst Chiral tetrahydrocarbazoles N-Tosyltetrahydrocarbazolone Enantioselective lactone synthesis DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.3) Synthesis, spectral characterization and biological evaluation of carbamate and sulfonamide derivatives of cis-tramadol
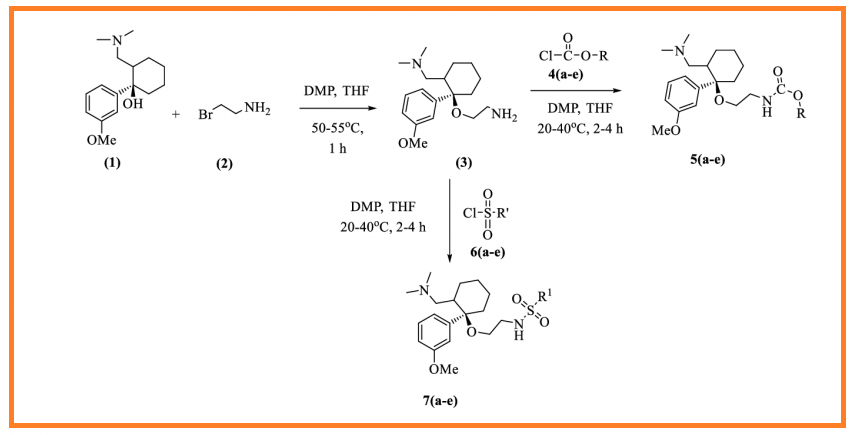
A series of new carbamates and sulfonamide derivatives of cis-tramadol 5(a-e) and 7(a-e) have been synthesized in high yields by the reaction of cis-tramadol with various chloroformates 4(a-e) and sulfonyl chlorides 6(a-e). All the newly synthesized compounds were characterized by IR, NMR (1H and 13C), mass and elemental analyses. The synthesized compounds were evaluated for their antibacterial and antifungal activities. Compounds 5a, 5b, 7a and 7c exhibited potent antibacterial and antifungal activity.
DOI http://doi.org/10.25135/acg.oc.157.2307.2834 Keywords cis-Tramadol carbonyl chloridates sulfonyl chlorides antibacterial antifungal activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.4) Novel oxalamide derivatives for COXs expression and breast cancer: design, synthesis, biological evaluation, and docking studies
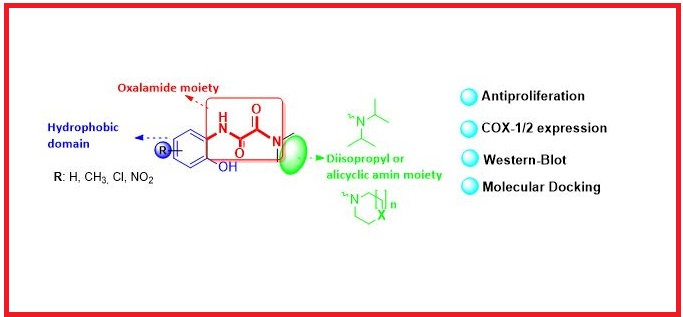
IIn the present study, new oxalamide-based compounds were designed from thalidomide and synthesized easily and with high yields (from 69% up to 93%) by a two-step method. The antiproliferative effects of synthesized 6a-d and 7a-d compounds on (ER+) MCF-7 and (ER-) MDA-MB-231 breast cancer cell line and human fibroblast WI-38 healthy cell line were investigated by the MTT method. The results showed that compound 7d was the most potent candidate against both MCF-7 and MDA-MB-231 cell lines with IC50 = 4.72 µM and 6.37 µM, respectively. To investigate whether antiproliferative effect of the compounds on breast cancer cell lines is dependent on COXs, expressions of COX-1/2 on the MCF-7 cell line were investigated by the Western-Blot technique. Among synthesized compounds, compound 7d increased the expression of both COX-1 and COX-2. The inhibition potential of compounds on COX-1/2 enzymes was investigated by molecular docking compared to inhibitor co-ligand celecoxib in crystal structures of COX-1 (PDB ID: 3KK6) and COX-2 (PDB ID: 3LN1). Docking results indeed showed that compound 7d had a higher binding affinity for both COX-1 and COX-2 active sites. Consequently, the novel oxalamide-based compounds presented here may be important candidate molecules for the development of new COX-dependent antiproliferative agents.
DOI http://doi.org/10.25135/acg.oc.154.2306.2820 Keywords Oxalamides antiproliferation western-blotting COXs expression molecular docking DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.5) Molecular modeling, synthesis and characterization of FOLR1 specific peptides for tumor targeting activity
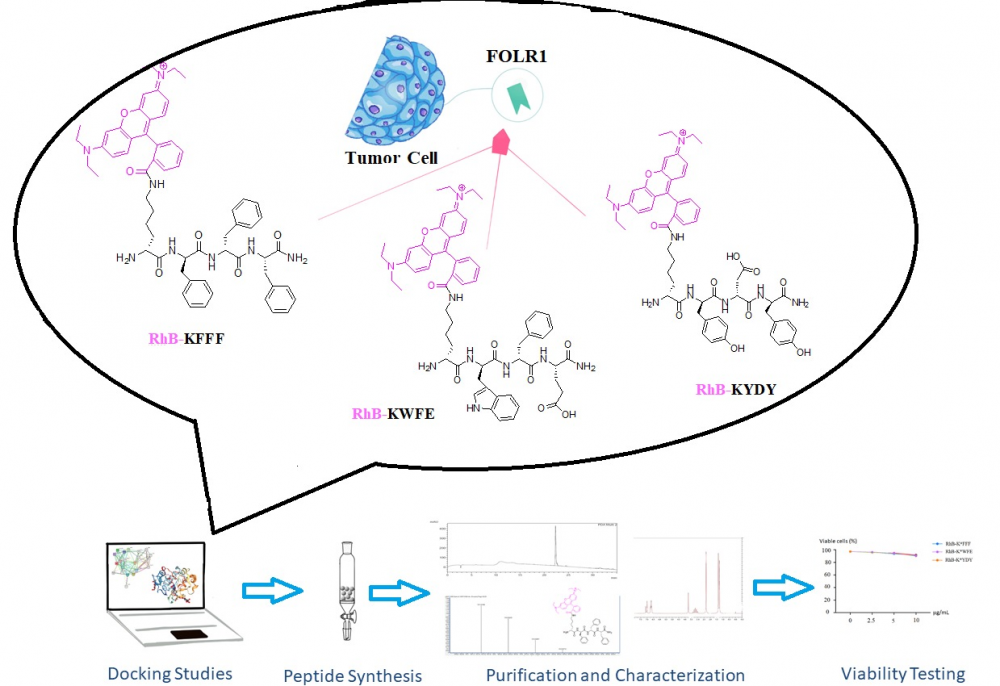
Peptides have low oral bioavailability, low plasma stability, and short circulation time; therefore, they are used in targeted strategies in cancer. In this study, according to in silico analysis, novel small peptide sequences, which are consist of three amino acid residues, with high binding capacity against the human FOLR1 surface molecule were obtained. Modeling studies were carried out to determine peptide sequences. RhB-K*FFF, RhB-K*WFE, and RhB-K*YDY peptides have been synthesized by using the Solid Phase Peptide Synthesis (SPPS) method, purified by Reverse Phase High-Performance Liquid Chromatography (RP-HPLC) and characterized by Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS/MS) and proton nuclear magnetic resonance (1H-NMR). The purity of RhB-K*FFF, RhB-K*WFE, and RhB-K*YDY peptide are 96%, 95%, and 92%, respectively. Also, cell viability test was performed for the peptides. In our further study, the peptide with highest binding affinity will be conjugated with chemotherapeutic agent in order to improve its anti-cancer activity.
DOI http://doi.org/10.25135/acg.oc.158.2308.2887 Keywords Cancer FOLR1 protein molecular modeling peptide synthesis DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.6) Erratum to “Facile microwave synthesis of a novel phenothiazine derivative and its cytotoxic activity” [Org. Commun. 13:4 (2020) 175-183]
It was wrongly indicated in the Acknowledgement section that a Turkish patent was received for 3-(2-methylthio10H-phenothiazine-10-yl-propyl)-pyrimidine-2,4-(1H,3H)-dione. This should be corrected as “For its novelty and great cytotoxic activity, a Turkish patent was applied (Applicationt ID: 2013/139199) for 3-(2-methylthio10H-phenothiazine-10-yl-propyl)-pyrimidine-2,4-(1H,3H)-dione. The patent application has still been under evaluation”. The author apologizes for this mistake
DOI http://doi.org/10.25135/acg.oc.148.2303.28001 Keywords Erratum phenothiazine derivatives DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.