Organic Communications
Year: 2023 Volume: 16 Issue:4 October-December
1) Water extract of onion: chemoselective synthesis of 2-substituted benzimidazole derivatives
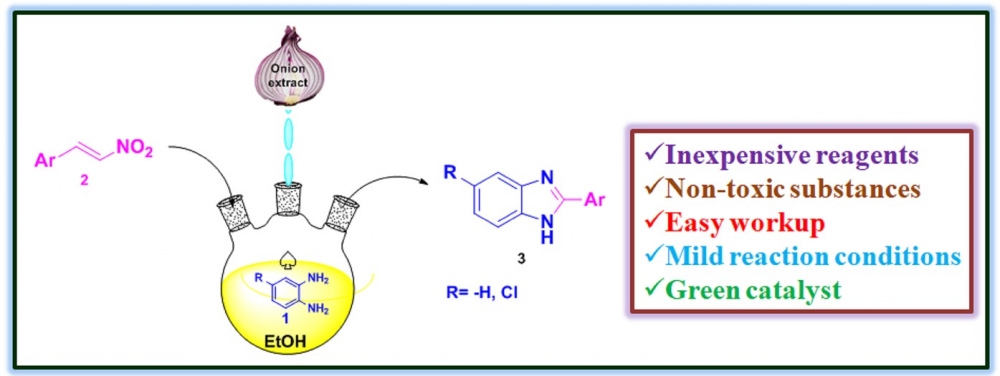
A facile, efficient, green synthetic route was developed for the selective synthesis of 2-substituted benzimidazole derivatives via the reaction of 1,2-diamines with aromatic, aliphatic and hetero aromatic nitroalkenes in the presence of an onion extract as a green catalyst. In this reaction, fifteen 2-substituted benzimidazole derivatives were obtained in good to excellent yields (80-95%). The method presented here offers several benefits, including minimal energy usage, cheap, nontoxic catalyst, simple workup procedure and without column purification. The usage of onion extract creates this approach is environmentally friendly and makes a significant addition to the currently available techniques for the creation of 2-substituted benzimidazoles. Further, the scope and constraints were also investigated, and a conceivable reaction mechanism was put forth.
DOI http://doi.org/10.25135/acg.oc.157.2305.2790 Keywords Chemoselectivity water extract of onion green catalyst 2-substituted benzimidazoles 1,2-Diamines DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.2) An efficient synthesis of quinoxaline derivatives using HCTU as catalyst in DMF
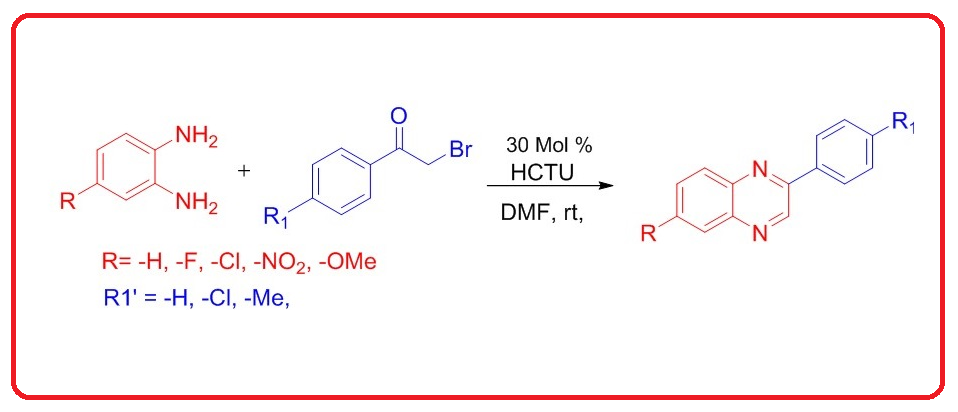
We have successfully developed a novel and efficient method for the synthesis of quinoxaline and its derivatives. This method involves the reaction between various 1,2-diamines and substituted phenacyl bromides in the presence of a catalytic amount of O-(1H-6-Chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HCTU) in dimethylformamide (DMF) as the solvent. This protocol offers several advantages, including mild reaction condition, short reaction time and moderate to high yield of the desired product(s).
DOI http://doi.org/10.25135/acg.oc.160.2308.2876 Keywords Quinoxalines HCTU 1,2-Diamines phenacyl bromides DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.3) Synthesis and bioactivity of 1-substituted tetrahydroisoquinolines derived from phenolic aldehydes
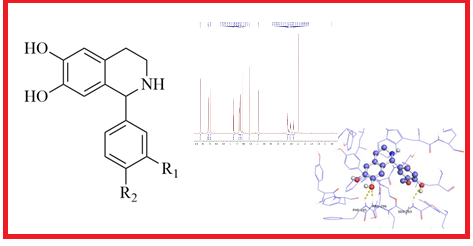
Phenolic aldehydes and their derivatives found in nature are well-known for their potential biological activity. In this study, four 1-substituted 1,2,3,4-tetrahydroisoquinolines (THIQs) derived from phenolic aldehydes were synthesized by phosphate buffer mediated Pictet-Spengler reaction. All derivatives were chemically and structurally characterized by elemental CHN analysis and spectroscopic methods (IR, HR-ESI-MS, 1H- and 13C-NMR). 1-Substituted THIQs derived from 3,4-dihydroxybenzaldehyde and 4-hydroxy-3-methoxybenzaldehyde were described for the first time. In order to cover the diversity of the mechanistic approach, but also to establish the relationship between structure and activity, antioxidant activity was examined by five different in vitro methods, namely: neutralization and reduction of stable free radicals 2,2-diphenyl-1-picrylhydrazyl and radical cation derived from [(2,2´-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)], ferric reducing antioxidant power, oxygen radical absorbance capacity, and ability to chelate Fe(II) ions. In vitro inhibition of acetylcholinesterase (AChE) was examined by the Ellman's colorimetric method, while computer-simulated docking was used to reveal the preferred binding site and major interaction between AChE and THIQs. Antibacterial testing was examined using the agar well method and results were presented in the form of zones of inhibition (mm).
DOI http://doi.org/10.25135/acg.oc.159.2310.2920 Keywords Synthesis bioactivity 1-Substituted tetrahydroisoquinolines phenolic aldehydes DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.4) Synthesis and antimicrobial activities of unsymmetrical thioditetrazoles and their precursor thiotetrazoles
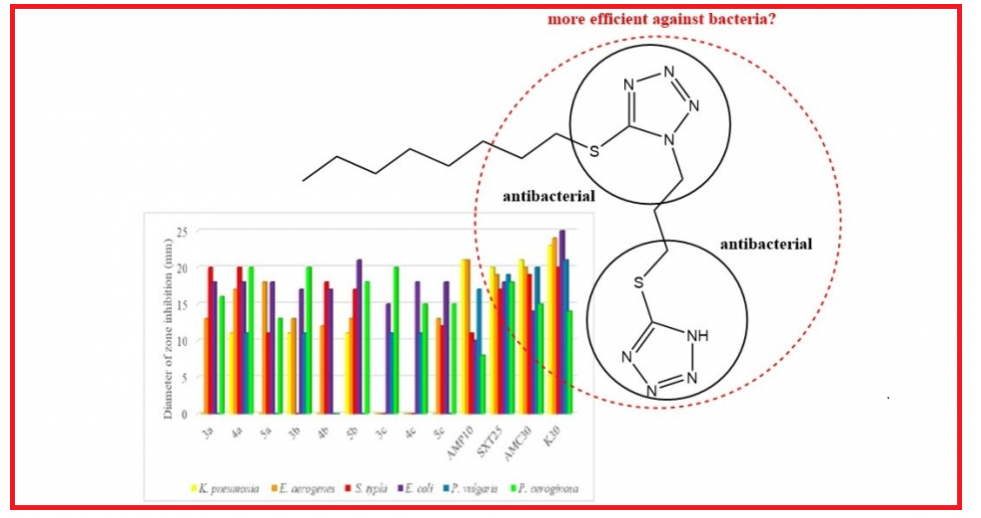
In this study, a synthetic route to access unsymmetrical ditetrazoles was developed. By using this method, the first examples of unsymmetrical thioditetrazole compounds were synthesized. In vitro, antibacterial efficacy studies were carried out for all the thioditetrazoles and their precursor thiotetrazoles against ten bacteria. The synthesized compounds showed a strong antibacterial effect against pathogenic Gram-negative bacteria, especially Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa (up to 21 mm).
.DOI http://doi.org/10.25135/acg.oc.158.2309.2893 Keywords Tetrazole thiotetrazole dithiotetrazole antibacterial activity antifungal activity DETAILS PDF OF ARTICLE © 2023 ACG Publications. All rights reserved.